Abstract
Autoimmunity and immune dysregulation may lead to cytopenia and represent key features of many primary immunodeficiencies (PIDs). Especially when cytopenia is the initial symptom of a PID, the order and depth of diagnostic steps have to be performed in accordance with both an immunologic and a hematologic approach and will help exclude disorders such as systemic lupus erythematosus, common variable immunodeficiency, and autoimmune lymphoproliferative syndromes, hemophagocytic disorders, lymphoproliferative diseases, and novel differential diagnoses such as MonoMac syndrome (GATA2 deficiency), CD27 deficiency, lipopolysaccharide-responsive beige-like anchor (LRBA) deficiency, activated PI3KD syndrome (APDS), X-linked immunodeficiency with magnesium defect (MAGT1 deficiency), and others. Immunosuppressive treatment often needs to be initiated urgently, which impedes further relevant immunologic laboratory analyses aimed at defining the underlying PID. Awareness of potentially involved disease spectra ranging from hematologic to rheumatologic and immunologic disorders is crucial for identifying a certain proportion of PID phenotypes and genotypes among descriptive diagnoses such as autoimmune hemolytic anemia, chronic immune thrombocytopenia, Evans syndrome, severe aplastic anemia/refractory cytopenia, and others. A synopsis of pathomechanisms, novel differential diagnoses, and advances in treatment options for cytopenias in PID is provided to facilitate multidisciplinary management and to bridge different approaches.
Introduction
Primary immunodeficiencies (PIDs) are classified into nine subclasses, depending on their underlying immunologic defect or predominant symptom.1-3 The current view of PIDs includes an increasing number of syndromes that are associated with immune dysregulation and autoimmunity as a predominant feature rather than an overt pathologic risk of infections. Cytopenia, defined as the reduction of one or more mature blood cell types (eg, neutropenia, anemia, or thrombocytopenia) in the peripheral blood, may be a typical first symptom of such an immunodeficiency. Possible causes of cytopenia in PIDs comprise cellular or humoral autoimmunity, immune dysregulation in form of hemophagocytosis or lymphoproliferation with or without splenic sequestration, bone marrow failure and myelodysplasia, or secondary myelosuppression. In some patients, cytopenia may be detected as an incidental finding, whereas other patients may be severely ill. Because primary defects in the number or function of phagocytes are classified under their own group of PIDs,3 the syndromes of severe congenital neutropenia (based on defects in ELANE,GFI1, HAX1, G6PC3, VPS45, and CSFR3 genes, or activating mutations in the Wiskott-Aldrich syndrome [WAS] gene)4-6 and cytopenia-linked metabolic diseases are not included in this overview. Similarly, isolated lymphopenia syndromes are excluded if they present without neutropenia, anemia, or thrombocytopenia; also excluded are non-PID inherited bone marrow failure syndromes such as Fanconi anemia, congenital amegakaryocytic thrombocytopenia, bone marrow failure with radioulnar synostosis, and others (Table 1 and footnotes). These syndromes are beyond the scope of this review because they do not represent a concurrence of immunodeficiency with cytopenia nor do they harbor an underlying defect of the immune system.
Like the self-limited benign forms of post- or parainfectious autoimmune cytopenia or acquired autoimmune neutropenia of childhood that typically occur independently of a (recognized) underlying PID, many but importantly not all cytopenias in patients with underlying PIDs are mediated by autoantibodies. Thus, it is essential that clinicians take an underlying PID into account in patients with clear antibody-mediated cytopenia and also in other situations as described. This review provides a conceptual synopsis of cytopenias in PIDs and aims to increase the awareness of hematologists as well as immunologists for this manifestation of PID.
Pathomechanisms of cytopenia in PID
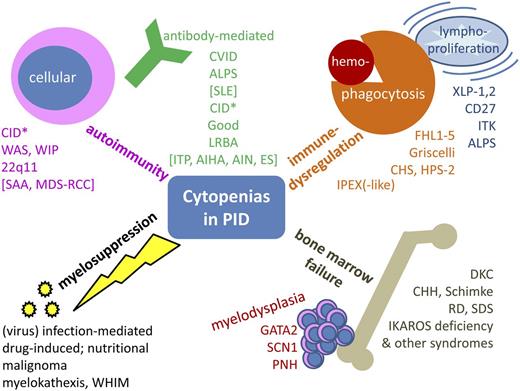
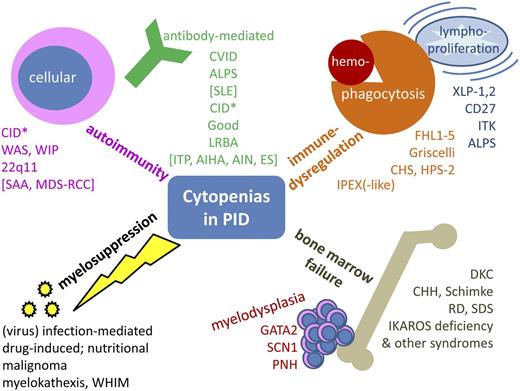
Cytopenia in PID may have a variety of causes. In some instances, it is a primary feature of the immunodeficiency, and in others, it is a secondary phenomenon. This review will focus on the clinical relevance of cytopenias and suggest the following grouping: (1) classic autoimmune cytopenias, further subdivided into autoantibody-mediated and cellular autoimmunity; (2) cytopenias in the context of immune dysregulation, lymphoproliferation, and inflammation in PID; (3) PID with bone marrow failure; and (4) toxic or infectious myelosuppression secondary or concomitant to PID (Figure 1).
Synopsis of cytopenias in PID. Conceptual overview, excluding primary defects of phagocyte number or function, inherited non-PID bone marrow failure syndromes, and disorders of isolated lymphopenia (without other cytopenia). *Includes hypomorphic mutations in SCID genes, CD40, CD40L, and other combined immunodeficiencies such as radiosensitive disorders, defects in the Ca++ channel, and activating PI3K syndrome. AIHA, autoimmune hemolytic anemia; AIN, autoimmune neutropenia; CHH, cartilage hair hypoplasia; CHS, Chediak-Higashi syndrome; DKC, dyskeratosis congenita; FHL1-5, familial hemophagocytic lymphohistiocytosis 1-5; HPS-2, Hermansky-Pudlak syndrome 2; ITK, IL-2–inducible T-cell kinase deficiency; LRBA, lipopolysaccharide-responsive beige-like anchor deficiency; PNH, paroxysmal nocturnal hemoglobinuria; RCC, refractory cytopenia of childhood; RD, reticular dysgenesis; SCN1, severe congenital neutropenia 1; SDS, Shwachman-Diamond syndrome; WHIM, warts, hypogammaglobulinemia, immunodeficiency, myelokathexis; WIP, WAS protein-interacting protein; XLP-1,2, X-linked lymphoproliferative disease 1,2.
Synopsis of cytopenias in PID. Conceptual overview, excluding primary defects of phagocyte number or function, inherited non-PID bone marrow failure syndromes, and disorders of isolated lymphopenia (without other cytopenia). *Includes hypomorphic mutations in SCID genes, CD40, CD40L, and other combined immunodeficiencies such as radiosensitive disorders, defects in the Ca++ channel, and activating PI3K syndrome. AIHA, autoimmune hemolytic anemia; AIN, autoimmune neutropenia; CHH, cartilage hair hypoplasia; CHS, Chediak-Higashi syndrome; DKC, dyskeratosis congenita; FHL1-5, familial hemophagocytic lymphohistiocytosis 1-5; HPS-2, Hermansky-Pudlak syndrome 2; ITK, IL-2–inducible T-cell kinase deficiency; LRBA, lipopolysaccharide-responsive beige-like anchor deficiency; PNH, paroxysmal nocturnal hemoglobinuria; RCC, refractory cytopenia of childhood; RD, reticular dysgenesis; SCN1, severe congenital neutropenia 1; SDS, Shwachman-Diamond syndrome; WHIM, warts, hypogammaglobulinemia, immunodeficiency, myelokathexis; WIP, WAS protein-interacting protein; XLP-1,2, X-linked lymphoproliferative disease 1,2.
Autoimmune-mediated cytopenia in PID
According to the causal involvement of autoantibodies against hematopoietic cells or predominant cellular cytotoxicity, the autoimmune-mediated cytopenias may be further subgrouped into antibody-mediated and cellular autoimmunity (Figure 1, upper left quadrant).
Autoantibody production may occur in B-cell–intrinsic defects or in disorders with disturbed T-cell–B-cell interaction and regulation. When B-cell maturation is impaired, vital mechanisms of B-cell tolerance induction, such as central and peripheral checkpoints of B-cell receptor generation to eliminate autoreactive clones, may be defective.7,8 Depending on the impaired maturation step and the affected pre-B-cell subtype, this may lead to hypogammaglobulinemia, at least to a specific antibody formation defect (eg, against polysaccharide antigens) and is often linked to the presence of autoreactive B cells. Common variable immunodeficiency (CVID), along with autoimmune lymphoproliferative syndrome (ALPS; the second most typical PID associated with autoantibody-mediated cytopenias),9-11 is the result of impaired B-cell maturation; both clinical and immune phenotypical classifications have been established as diagnostic criteria and to distinguish between CVID subgroups.12-14 ALPS represents a group of disorders with a primary defect in T-cell development and apoptosis that secondarily affects B cells and causes antibody-mediated autoimmunity.15,16 In addition to these two entities, overlapping syndromes have been reported between CVID and ALPS (with ALPS-linked increased T-cell receptor α/β- positive CD4– and CD8− double-negative T [DNT] cells in CVID patients or reduced CD27+ immunoglobulin D [IgD]+ and CD27+IgD– memory B cells and hypogammaglobulinemia as in CVID in ALPS patients).17 One of the novel PIDs in this subgroup of autoantibody-mediated cytopenia is lipopolysaccharide-responsive beige-like anchor deficiency, a rare B-cell defect involving autophagy and apoptosis that, along with immune cytopenia, is often linked to inflammatory bowel disease, multiorgan autoimmune phenomena, severe infections, and hypogammaglobulinemia18-20 [Markus G. Seidel, Tatjana Hirschmugl, Wolfgang Schwinger, Laura Gamez-Diaz, Nina Serwas, Andrea Deutschmann, Gregor Gorkiewicz, Werner Zenz, Christian Windpassinger, Bodo Grimbacher, Christian Urban, and Kaan Boztug; manuscript submitted July 2014]. Although the pathomechanism of this novel disease is incompletely understood, in vitro and in vivo immunologic analyses and human clinical data point toward a B-cell intrinsic defect in lipopolysaccharide-responsive beige-like anchor deficiency.18-20 An example of impaired T-cell–B-cell interaction is CD40/CD40L deficiency, in which the missing signal from T cells causes humoral autoimmunity as well as other severe immunologic symptoms; the phenotype for this deficiency is classified as combined immunodeficiency (CID).3,21
In addition to these primary or secondary humoral defects, intrinsic defects in T-effector cells may lead to cellular autoimmunity. The simplified principle is similar to that of B-cell maturation defects described above, namely that impaired T-cell development may lead to a lack of functional effectors against non-self or dangerous antigens and simultaneously yield “uneliminated” autoreactive clones with T-cell receptors directed against self-antigens. Defects in signaling or in T-cell receptor recombination or editing may result in both a deficit of FOXP3-positive regulatory T cells (Tregs) and an incomplete development of autoimmune regulator transcription factor–expressing medullary thymus epithelial cells that result in peripheral and central tolerance defects.22 Thus, many classic T-cell disorders such as combined immunodeficiencies (CIDs) that lack naïve T cells based on hypomorphic mutations in genes usually associated with severe CID (SCID; ie, leaky SCIDs such as RAG1, RAG2, adenosine desaminase, artemis, and purine nucleoside phosphorylase23-27 ) and well-known syndromes with immunodeficiency such as WAS, WAS protein-interacting protein deficiency, and 22q11 microdeletion syndrome may show some extent of autoimmunity as a result of autoreactive T cells and reduced T-cell regulation. Furthermore, certain T-cell signaling defects that may cause SCID or CID, such as ORAI-1, STIM-1, MAGT1, STK4, or LCK deficiencies as well as activating mutations of PI3KD, predispose to autoimmunity including cytopenias.3,28-32 Recently, loss of function of tripeptidyl peptidase 2 was demonstrated to cause CID with autoimmune cytopenia (manuscript by Polina Stepensky, Anne Rensing-Ehl, Ruth Gather, Shoshana Revel Vilk, Ute Fischer, Schafiq Nabhani, Sebastian Fuchs, Simon Zenke, Elke Firat, Vered Molho Pessach, Arndt Borkhardt, Mirzokhid Rakhmanov, Baerbel Keller, Klaus Warnatz, Hermann Eibel, Gabriele Niedermann, Orly Elpeleg, and Stephan Ehl, submitted August 2014; Hambleton et al33 ). In WAS, however, the basis of thrombocytopenia and microplatelets is the underlying cytoskeletal dysfunction.34,35 A close connection between T-cell and B-cell pathology exists (eg, in 22q11 syndrome and WAS) in which, in addition to T-cell dysfunction, there is also an altered B-cell differentiation/maturation pattern with predisposition to humoral autoimmunity.36,37 Conversely, PIDs with humoral autoimmune mechanisms that have historically been considered the principal cytopenia-linked PIDs, such as ALPS or CVID, have been shown to have impaired T-cell maturation38 or reduced Treg function,39-41 respectively.
Many hematologic conditions such as refractory cytopenia of childhood (RCC), myelodysplastic syndrome (MDS), severe aplastic anemia (SAA), chronic immune thrombocytopenia (cITP), Evans syndrome (ES), and/or rheumatologic diseases such as systemic lupus erythematosus (SLE) (shown in square brackets in Figure 1) are either the result of an unrecognized PID or are based on a variety of polygenetic or epigenetic defects in hematopoietic stem cells or within the immune system that in turn lead to autoimmune reactions. Both SAA and RCC/MDS, if no cytogenetic aberration or clonal evolution is detected, are being treated with T-cell–directed immunosuppression or hematopoietic stem cell transplantation, indirectly confirming a primary or secondary involvement of autoreactive T cells in pathogenesis.42-45 A variety of pathomechanisms underlying the antiplatelet autoimmunity in cITP has been suggested, ranging from dysfunctional Tregs and lacking regulatory B cells to cytokine gene polymorphisms, disturbed antigen presentation, and autoreactive B cells.46-50 The detection of CVID- or ALPS-like immune phenotypical parameters in a subgroup of patients with ES has recently been reviewed.9 SLE is a descriptive symptom complex mainly ascribed to humoral autoimmunity that may arise in PIDs, typically caused by deficiencies in the classical complement pathway (eg, C1q, C1R, C1s, C2, C4, C5, C6, C7, C8A, and C8B).1,3 In addition to these recognized PIDs, there are SLE-CVID/SLE-CID overlap syndromes and SLE features in hyper-IgM syndromes, including constitutive mismatch repair defects.1,3,51,52 However, because the diagnostic algorithms are not standardized and because existing recommendations (such as those for cITP53 or those within RCC/SAA international treatment guidelines from the European Working Group for childhood MDS54 ) are often not sufficiently executed before initiating immunosuppressive treatment, a substantial number of unrecognized PIDs may be hidden among these allegedly hematologic disorders. International initiatives for prospective registries aim to establish and continuously update diagnostic, prognostic, and therapeutic algorithms for immune cytopenias (eg, the intercontinental cooperative ITP study group, the French Reference Center for Rare Diseases and Autoimmune Cytopenias of Childhood, and the German Pediatric Hematology-Oncology Working Group ITP/ES prospective studies).55,56
Practically all conditions within this first group of diseases may lead to cytopenia without any previous history of infections or autoimmunity and may present with mild or absent clinical symptoms. However, acute hemolytic anemia, as well as newly diagnosed immune thrombocytopenia, are potentially life-threatening conditions.
Immune dysregulation underlying cytopenia in PID
One classical PID with immune dysregulation linked to cellular autoimmunity is immune dysregulation, polyendocrinopathy, and enteropathy X-linked (IPEX) syndrome, which is the result of a lack of functional FOXP3-positive Tregs that leads to a peripheral T-cell tolerance defect and often to cytopenia.57-59 In addition to the mutations in FOXP3 that cause IPEX,60,61 other components of the Treg activation pathway may be defective and may thus reduce the function and/or number of Tregs and lead to an IPEX-like syndrome (eg, deficiency of CD25 or STAT5b and gain-of-function mutations in STAT1).62-65 Interestingly, the classical central T-cell tolerance defect, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome ([APECED] due to a defect of the autoimmune regulator transcription factor) is not typically associated with cytopenias.66 Furthermore, immune dysregulatory processes such as hemophagocytosis or lymphoproliferation (and subsequent splenic sequestration of blood cells) may cause secondary cytopenia in critically ill patients. The underlying pathomechanisms are pathological macrophage activation, functional natural killer (NK) cell defects, and polyclonal or oligoclonal lymphoproliferation. These dysfunctions may arise as complications of certain infections, oncologic treatments including hematopoietic stem cell transplantation (often referred to as infection-associated macrophage-activation syndrome or secondary hemophagocytic lymphohistiocytosis67 or lymphoproliferative syndrome [with unknown genetic predisposition]), or as a main symptom of PIDs that stem from monogenetic communication defects between B and T cells. The manifestation of lymphoproliferative disorders is often triggered by primary Epstein-Barr virus (EBV) infection and is associated with a lack of invariant T-cell receptor NKT (iNKT) cells (eg, X-linked lymphoproliferative syndrome, CD27 deficiency, and interleukin-2 (IL-2)–inducible kinase deficiency). Likewise, interaction defects between T cells and the innate immune system are found in this category (familial hemophagocytic lymphohistiocytosis and immunodeficiencies with hypopigmentation; Figure 1, upper right quadrant) (Alkhairy O, Perez-Becker R, Driessen G, et al, manuscript submitted June 2014).67-71 Another newly identified PID with increased susceptibility to EBV-induced lymphoproliferation and development of lymphoma—the X-linked immunodeficiency with magnesium defect, EBV infection, and neoplasia syndrome, which is due to a magnesium transporter MAGT1 defect—also appears to predispose to autoimmune cytopenia,30 most likely as a result of autoantibodies generated on the basis of the underlying T-cell defect similar to the Ca-signaling defects ORAI-1 and STIM-1 (see above). ALPS should be mentioned again in this context, because splenic sequestration contributes to cytopenia in ALPS as in other lymphoproliferative syndromes (and sometimes also in CVID).
Bone marrow failure in PID
If pancytopenia is the initial clinical symptom, diagnostic algorithms in hematology-oncology exclude malignoma, RC/MDS, acquired and inherited bone marrow failure syndromes such as paroxysmal nocturnal hemoglobinuria, and Fanconi anemia, Shwachman-Diamond syndrome, and dyskeratosis congenita, and may end up with a diagnosis of exclusion such as SAA (depending on bone marrow cellularity, morphology, and cytogenetics), but do not always consider immunodeficiencies as the underlying cause. Because rare and novel PIDs such as immune osseous dysplasias (cartilage hair hypoplasia, Schimke syndrome) or MonoMac syndrome (GATA2 deficiency) may cause bone marrow failure and are not widely taken into consideration, these entities need to be mentioned here (Figure 1, lower right quadrant; Table 1).3,72-74 In contrast to the widespread view of a PID diagnosis being dependent on compromised immunity, patients with GATA2 deficiency, as with other PID-linked cytopenias, may be asymptomatic and may lack a history of severe infections.74 Because of its presentation as SCID with granulocytopenia or pancytopenia and deafness, reticular dysgenesis (deficiency of AK2) is unlikely to be missed during a differential diagnosis. The deficiency of IKAROS, a zinc finger transcription factor essential during hematopoiesis,75 has been reported to be associated with hematologic malignancies (reviewed in Wang et al76 ) and also with congenital pancytopenia in humans.77 It is known to impede B- and NK-cell development and is thus suspected to cause an immunodeficiency with antibody deficiency and cytopenia3 (reviewed in John and Ward78 ).
Secondary myelosuppression in PID
Unspecific secondary bone marrow suppression may occur in PID as in secondary states of immunosuppression due to viral (or rarely bacterial) infections, toxic marrow damage from drugs used to treat infections or autoimmunity in PID, extrusion and/or suppression of hematopoiesis by malignant cells, or simply in states of nutritional deficiencies (eg, resulting from inflammatory bowel disease, metabolic disorders, or wasting conditions involving vitamin B12, folate, or iron; Figure 1, lower left quadrant). Myelokathexis (trapping of neutrophils in the bone marrow) is a rare condition involving pseudosuppression of the marrow in which the marrow is unable to release mature neutrophils into the periphery because of a gain-of-function mutation in CXCR479 ; this PID (warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis [WHIM] syndrome) is usually diagnosed based on the typical symptoms indicated in its name. X-linked agammaglobulinemia is not typically linked with cytopenia; however, it is listed here because some patients experience neutropenia, which may be an underestimated clinical concern; its mechanisms include enhanced neutrophil apoptosis and have recently been demonstrated.80
Management
The following recommendations should be considered as general background information for the differential diagnostic workup and consideration of treatment options for cytopenia in the context of PIDs. They are not guaranteed to be complete for any individual situation nor are they designed for hematologic emergency situations or for managing forms of cytopenia other than those associated with PID (such as hemoglobinopathies). This review should not and cannot replace a consultation with a pediatric hematologist or hematologist-oncologist and a pediatric immunologist who can perform the differential diagnostic procedures for PIDs associated with cytopenia. Likewise, this review cannot provide a general diagnostic algorithm or therapeutic guideline because the spectrum of possible underlying diseases is too vast and heterogeneous for one tool to suffice.
Diagnostic analyses
In PID-associated cytopenia, the first question to answer is whether it is a result of the increased loss or the decreased production of blood cells. This may be an emergency situation because autoimmune hemolytic anemia may evolve into a life-threatening situation within hours. Therefore, the first laboratory analyses that should be performed are for cell lysis parameters (eg, potassium, lactate dehydrogenase, aspartate transaminase, uric acid); for anemia, additional parameters include those for hemolysis (indirect bilirubin, absolute reticulocyte count, haptoglobin) and immunologic and metabolic parameters (eg, direct and indirect Coombs test; IgG, IgA, and IgM; fluorescence-activated cell sorter analyses for T-, B-, and NK-cell counts; serum ferritin concentration; vitamin B12; and folate [soluble IL-2 receptor, IL-18, soluble Fas ligand]) (Table 1 and Sills81 ). Antiplatelet antibodies are of no help in the differential diagnostic process because they are present in less than two-thirds of patients with immune thrombocytopenia and are not predictive, specific, or prognostically relevant,82-84 whereas antierythrocyte (bound or soluble) and antigranulocyte antibodies have a rather high specificity for autoimmune hemolytic anemia and autoimmune neutropenia, respectively. Of note, the detection of antibodies against granulocyte surface antigens (not to be confused with antineutrophil cytoplasma antibodies) is a delicate analysis that depends on using specialized reference laboratories to perform tests and interpret results; it is not a test that can be performed under emergency conditions. Before an immunosuppressive treatment is initiated, at least some of the following special immunologic tests should be performed to exclude diseases that can not be easily diagnosed under intravenous immunoglobulin or pharmacologic immunosuppression. Parameters that are impacted by intravenous immune globulin are mainly the serologic tests such as quantitative immunoglobulins, antibodies against vaccination antigens and previous infectious diseases (protein and polysaccharide antigens), and isohemagglutinins. Analyses that should be done before pharmacologic immunosuppression (including the use of corticosteroids) are immune cellular tests such as quantification of T-, B-, and NK cells, T-cell receptor α/β-positive CD4– and CD8− double-negative T cells, CD27+IgD+ and CD27+IgD– memory B cells; functional assays such as in vitro lymphocyte proliferation, NK/cytolytic T lymphocyte cytotoxicity; and CD107a degranulation assays to exclude functional T- or NK-cell defects. If a primary lymphoproliferative disorder is suspected, invariant T-cell receptor NKT (iNKT) cells should be quantified (Table 1). Infection serology should be analyzed for EBV, cytomegalovirus, parvovirus B19, and other DNA viruses, as well as HIV and hepatitis viruses; and if an antibody formation defect is suspected, a virus nucleic acid detection test may be needed. In many cases, bone marrow smears and trephine biopsies will need to be assessed and, ideally, they should be sent to reference laboratories for evaluation, as is done in international treatment optimization studies. More specific laboratory tests and genetic analyses depend on the clinical situation, the immune hematologic phenotype, and patient’s history, as outlined in Table 1.
Treatment options
The main intention of this review is to increase awareness of and to classify the types of cytopenia that occur in the context of PID to facilitate correct management. Table 1 provides an overview of typical and potential treatment approaches. However, it is not feasible to provide general treatment guidelines for cytopenias in PID because of the heterogeneity of underlying causes and mechanisms, as outlined above and in Figure 1 and Table 1. Although PIDs with autoimmune-mediated cytopenia most often respond well to various degrees and modes of immunosuppression (recently reviewed by Teachey and Lambert11 ), certain diseases might represent an indication for early hematopoietic stem cell transplantation, which should be performed according to international transplantation guidelines (such as those recommended by the European Group for Blood and Marrow Transplantation85 ) and within well-controlled clinical trials. Rituximab is used when autoreactive CD20-expressing B-cell clones need to be eradicated and also in EBV-mediated immune dysregulation such as hemophagocytosis and lymphoproliferation (eg, in X-linked lymphoproliferative syndrome, CD27, and IL-2–inducible kinase deficiency), because B cells represent the main pool of EBV and are therefore the trigger for subsequent dysregulated immune processes. A novel group of substances for treating immune-mediated thrombocytopenia (and potentially also pancytopenia resulting from RC/MDS) are thrombopoietin receptor agonists such as romiplostim and eltrombopag.44,86-89 Although this treatment option appears even less causal than immunosuppression, at least short-term side effects are low and response rates are promising in adults and children. The future will tell whether long-term follow-up remains acceptable and whether the spectrum for clinical use of thrombopoietin receptor agonists will be extended. In the future, other novel substances for use in antibody-mediated cytopenias may include the proteasome inhibitor bortezomib, the anti-B-cell–activating factor antibody belimumab, the anti-IL-6–directed antibody tocilizumab, the anti-CD22 antibody epratuzumab, and an anti-APRIL antibody, which are currently used only within phase 1 to 3 clinical trials against refractory autoimmunity in certain indications such as in subgroups of patients with SLE, multiple sclerosis, other severe autoimmune diseases, in antibody-mediated graft rejection, or as a treatment adjunct in certain B-cell malignancies.90-92 The recommendation to avoid splenectomy, which is still one of the widely accepted (and likely least expensive) options for treating hypersplenism-associated thrombocytopenia associated with the risk of overwhelming post-splenectomy infection, is increasingly confirmed and corroborated by long-term follow-up data (eg, from ALPS patients in Price et al15 ).
In conclusion, this review provides a conceptual description of known and novel observations of cytopenias in PID and their management. Cytopenia may be the initial presenting symptom of patients with PID, irrespective of a previous history of severe infections, autoimmunity, or a familial predisposition. Because hematologic diagnostic procedures rarely include the differential diagnosis of PIDs and because clinical immunologists often have little experience in the management of newly diagnosed cytopenias, awareness of this challenging and growing field is critical. Hence, the hematologists’ and immunologists’ diagnostic approaches should be combined before immunosuppressive treatment is initiated, ideally within prospective clinical studies.
Acknowledgments
The author thanks all participants of the 2014 Annual Meeting of the Arbeitsgemeinschaft Pädiatrische Immunologie (German Working Group of Pediatric Immunology) for constructive criticism of figure and table drafts. Fruitful discussions on immune tolerance and inborn errors of hematopoiesis with A. Heitger and O.A. Haas, Vienna, Austria, are highly appreciated and have substantially contributed to this work. The author thanks C. Urban and the team of Pediatric Hematology-Oncology, Graz, for being supportive.
Authorship
Contribution: M.G.S. designed the concept of the article, drew the figure, and wrote the final draft.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Markus G. Seidel, Department of Pediatrics and Adolescent Medicine, Division of Pediatric Hematology-Oncology, Medical University Graz, Auenbruggerplatz 38, 8036 Graz, Austria; e-mail: markus.seidel@medunigraz.at.