Abstract
As immune-based therapies for cancer become potent, more effective, and more widely available, optimal management of their unique toxicities becomes increasingly important. Cytokine release syndrome (CRS) is a potentially life-threatening toxicity that has been observed following administration of natural and bispecific antibodies and, more recently, following adoptive T-cell therapies for cancer. CRS is associated with elevated circulating levels of several cytokines including interleukin (IL)-6 and interferon γ, and uncontrolled studies demonstrate that immunosuppression using tocilizumab, an anti-IL-6 receptor antibody, with or without corticosteroids, can reverse the syndrome. However, because early and aggressive immunosuppression could limit the efficacy of the immunotherapy, current approaches seek to limit administration of immunosuppressive therapy to patients at risk for life-threatening consequences of the syndrome. This report presents a novel system to grade the severity of CRS in individual patients and a treatment algorithm for management of CRS based on severity. The goal of our approach is to maximize the chance for therapeutic benefit from the immunotherapy while minimizing the risk for life threatening complications of CRS.
Introduction
Cancer immunotherapy seeks to harness the power of the immune system to eradicate malignant tissues. After decades of research, numerous cancer immunotherapies have shown definitive clinical efficacy, spanning graft-versus-leukemia to eradicate leukemia following hematopoietic stem cell transplantation (HSCT),1 monoclonal antibodies (mAbs) to improve survival for patients with B-cell lymphomas and HER2-expressing breast cancer,2,3 and a therapeutic cancer vaccine for hormone refractory prostate cancer.4 Recently, mAbs that block key checkpoints on T cells have improved survival in metastatic melanoma and induced antitumor effects in other cancers,5,6 bispecific mAbs have mediated impressive responses in B-cell acute lymphoblastic leukemia7 (ALL), and dramatic antitumor effects have been observed using adoptive T-cell immunotherapy, increasingly using genetic engineering to create tumor antigen-specific T cells.8-14
Risks associated with cancer immunotherapy can be broadly classified into autoimmune toxicity and cytokine-associated toxicity. Autoimmune toxicity, so-called “on target, off-tumor toxicity,” results from antigen-specific attack on host tissues when the targeted tumor associated antigen is expressed on nonmalignant tissue. Autoimmune toxicity occurs not uncommonly after treatment with checkpoint inhibitors5,6,15 and has resulted in fatal toxicities after infusion of genetically engineered T cells targeting MAGE-A3.16-18 Cytokine-associated toxicity, also known as cytokine release syndrome (CRS), is a non–antigen-specific toxicity that occurs as a result of high-level immune activation. The magnitude of immune activation typically required to mediate clinical benefit using modern immunotherapies exceeds levels of immune activation that occurring in more natural settings, and as immune-based therapies have become more potent, this syndrome is becoming increasingly recognized. This report reviews current concepts regarding the pathophysiology of CRS, describes the clinical manifestations of the syndrome, and provides a grading system to assess severity and guide treatment of CRS in the context of emerging immune-based therapies. Institutional Review Board protocols approved all of the studies that patients discussed in this paper were enrolled on, and studies were conducted in accordance with the Declaration of Helsinki.
Case 1: grade 1 CRS after CAR T-cell therapy for leukemia
An 11-year-old girl with primary refractory pre-B-cell ALL failed to obtain remission after 3 cycles of intensive chemotherapy and was referred for CD19–chimeric antigen receptor (CAR) therapy. At the time of enrollment, 58% of her marrow mononuclear cells were lymphoblasts. She received fludarabine and cyclophosphamide for lymphodepletion on days −4 through −2, and then 1 × 106 CD19 CAR T cells/kg on day 0. She developed fever on day 1 that peaked at 40.7°C associated with rigors, which persisted for 5 days. No source of infection was identified, and she remained hemodynamically stable with no evidence of neurologic dysfunction. Plasma interleukin (IL)-6 levels peaked at 222 pg/mL (∼30-fold over baseline). Assessment on day 28 revealed minimal residual disease-negative complete remission. She subsequently underwent matched sibling HSCT and remains disease free 16 months later.
Case 2: grade 3 CRS after genetically unmodified cytotoxic T cells (CTL) therapy for EBV-associated lymphoma
A 19-year-old woman with relapsed Epstein-Barr virus (EBV)-negative Hodgkin’s lymphoma received a 9/10 HLA DRB1 mismatched unrelated HSCT following conditioning with 600 cGy total body irradiation, fludarabine, and alemtuzumab. Rapidly progressive EBV-positive post-transplant lymphoproliferative disease (EBV-PTLD) developed 3 months after transplant with elevated EBV DNA (>100 000 copies/106 cells) and extensive nodal involvement on positron emission tomography. Biopsy confirmed EBV-PTLD. She experienced only a transient response following 2 doses of rituximab. A donor-derived, peptide rapidly generated multispecific (EBV, adenovirus, BK virus, and HHV6) CTL line was generated, and 5 × 106 cells/m2 were infused on a patient-specific investigational new drug application. EBV DNA levels fell to normal levels with clinical and radiological improvement within 2 weeks after infusion. Shortly afterward, she developed fever, rash, tachycardia, and hypotension with elevated IL-5, IL-13, IL-10, IL-6, and interferon (IFN)γ levels (range, 5- to 69-fold over baseline), associated with ≥10 000-fold expansion of EBV (EBNA1, LMP2, and BZLF1)-specific CTLs in the peripheral blood. She had no evidence of tumor lysis. She was treated with dopamine and norepinephrine, etanercept, and methylprednisolone (1 mg/kg/day × 2). Symptoms resolved within hours, and vasopressor support was discontinued. Cytokine levels returned to baseline within 3 weeks. Nine months after this CRS, her EBV lymphoma remains in remission.
Case 3: grade 3 CRS after CAR T-cell therapy for leukemia
An 11-year-old girl relapsed 6 months after HSCT for B-ALL and enrolled on a clinical trial of CD19-CAR therapy. She received cyclophosphamide and fludarabine for lymphodepletion: on day –1, her marrow showed 51% blasts, and on day 0, 2 × 107 CD19-CAR cells were administered. On day 1, she was admitted to the hospital with fever. Blood cultures grew Streptococcus mitis, and she became afebrile on vancomycin. On day 4, she developed a new fever with headache and nausea. On day 5, she developed tachycardia, hypotension (70/40 mm Hg), and a new oxygen requirement. She received 2 fluid boluses and was transferred to the pediatric intensive care unit, where she received dopamine and norepinephrine. She was treated with 8 mg/kg tocilizumab and within hours became afebrile and no longer required supplemental oxygen, and pressors were weaned to minimal levels. On day 7, she became febrile again and experienced intermittent hypotension requiring continued pressor support. Ferritin (130 000 ng/mL) and C-reactive protein (CRP; 21.5 mg/L) peaked on day 7. On day 9, she developed encephalopathy, with near aphasia, disorientation, and lethargy, which improved rapidly over the next 2 days without further immunosuppressive therapy. Pressors were discontinued on day 10, and encephalopathy resolved completely by day 13. She was discharged home on day 15, and her marrow demonstrated complete remission on day 28.
Pathophysiology
CRS clinically manifests when large numbers of lymphocytes (B cells, T cells, and/or natural killer cells) and/or myeloid cells (macrophages, dendritic cells, and monocytes) become activated and release inflammatory cytokines. CRS has classically been associated with therapeutic mAb infusions, most notably anti-CD3 (OKT3), anti-CD52 (alemtuzumab),19 anti-CD20 (rituximab), and the CD28 super-agonist, TGN1412.20 In these settings, symptom onset typically occurs within minutes to hours after the infusion begins.20-22 CRS has also recently been reported following administration of bi-specific antibodies for leukemia,23 infusion of haploidentical mononuclear cells to patients with refractory leukemia,24 and adoptive immunotherapies for cancer, most notably T cells engineered to express CARs.8,25-27
Severe CRS induced in 6 healthy subjects who received TGN1412 provides valuable insights into the pathophysiology of this syndrome without the background of malignant disease.20 All subjects received a single dose of TGN1412 on the same day, within minutes of one another. Symptoms began approximately 1 hour after the infusion and coincided with peak cytokine levels. Tumor necrosis factor (TNF)α rose first, followed by IFNγ, IL-1β, IL-2, IL-6, IL-8, and IL-10. Within 24 hours, all subjects required transfer to an intensive care unit for management of respiratory distress and renal dysfunction that ultimately required dialysis. Following symptom onset on day 1, all subjects were treated with corticosteroids, and within 2 to 3 days, cytokines returned to normal levels. Two of the subjects experienced prolonged organ dysfunction well beyond the return of cytokine levels to baseline.
Timing of symptom onset and CRS severity depends on the inducing agent and the magnitude of immune cell activation. CRS following rituximab for CD20+ malignancies typically occurs within minutes to hours, and patients with >50 × 109/L circulating lymphocytes have increased rates of CRS symptoms.22 In recent reports of CRS following adoptive T-cell therapy for cancer,8,10,14,28 the incidence and severity of the syndrome also appears greater when patients have large tumor burdens, presumably because this leads higher levels of T-cell activation. In contrast, symptom onset typically occurs days (cases 1 and 3) to occasionally weeks (case 2) after the T-cell infusion, coinciding with maximal in vivo T-cell expansion. Like CRS associated with mAb therapy, CRS associated with adoptive T-cell therapies has been consistently associated with elevated IFNγ, IL-6, and TNFα levels, and increases in IL-2, granulocyte macrophage–colony-stimulating factor (GM-CSF), IL-10, IL-8, IL-5, and fracktalkine have also been reported.8,10,26,28 A clear cell dose:response relationship for CRS related to adoptive T-cell therapies has been difficult to define, but very high doses of T cells may result in earlier onset, because 1 case report of a fatal event following infusion of 1010 CAR-modified T cells bore hallmarks of CRS and had rapid onset within minutes of infusion.25
Emerging evidence implicates IL-6 as a central mediator of toxicity in CRS. IL-6 is a pleiotropic cytokine with anti-inflammatory and proinflammatory properties. IL-6 signaling, illustrated in Figure 1, requires binding to cell-associated gp130 (CD130), which is broadly expressed, and the IL-6 receptor (IL-6R) (CD126).29 IL-6R is cell associated on macrophages, neutrophils, hepatocytes, and some T cells and mediates classic signaling, which predominates when IL-6 levels are low. However, when IL-6 levels are elevated, soluble IL-6R can also initiate trans-signaling, which occurs on a much wider array of cells.30 Current models hold that anti-inflammatory properties of IL-6 are likely mediated via classic signaling, whereas proinflammatory responses occur as a result of trans-signaling. High levels of IL-6, present in the context of CRS, likely initiates a proinflammatory IL-6-mediated signaling cascade.
IL-6 signaling and inhibition by tocilizumab. (A) Classic IL-6 signaling restricted to IL-6R–expressing cells shown in pink (macrophages, neutrophils, T cells, and hepatocytes), which predominates when IL-6 levels are low. IL-6 binds to cell-associated IL-6R, leading to homodimerization of gp130 and initiation of downstream pathways. (B) Both classic and trans-IL-6 signaling, which occurs when IL-6 levels are elevated, leading to IL-6 signaling on a broad array of cells, because gp130 is ubiquitously expressed. Tocilizumab binding to both cell-associated IL-6R and soluble IL-6R inhibits classic and trans-signaling. IL-6R–expressing cells are shown in pink, whereas non-IL-6R–expressing cells are shown in blue.
IL-6 signaling and inhibition by tocilizumab. (A) Classic IL-6 signaling restricted to IL-6R–expressing cells shown in pink (macrophages, neutrophils, T cells, and hepatocytes), which predominates when IL-6 levels are low. IL-6 binds to cell-associated IL-6R, leading to homodimerization of gp130 and initiation of downstream pathways. (B) Both classic and trans-IL-6 signaling, which occurs when IL-6 levels are elevated, leading to IL-6 signaling on a broad array of cells, because gp130 is ubiquitously expressed. Tocilizumab binding to both cell-associated IL-6R and soluble IL-6R inhibits classic and trans-signaling. IL-6R–expressing cells are shown in pink, whereas non-IL-6R–expressing cells are shown in blue.
Clinical symptomatology
Symptomatology and severity associated with CRS varies greatly (Table 1), and management can be complicated by intercurrent conditions in these patients. Fever is a hallmark, and many features of CRS mimic infection. It is not uncommon for patients to experience temperatures exceeding 40.0°C. Hence, infection must be considered in all patients presenting with CRS symptoms after appropriate cultures are obtained and empiric antibiotic therapy is initiated. This is of even greater importance when patients are neutropenic. Indeed, 1 death reported following anti-CD19 CAR-based T-cell therapy occurred coincident with a syndrome resembling CRS, wherein the patient had infection predating the T-cell infusion that may have contributed to the fatal outcome.27
Potentially life-threatening complications of CRS include cardiac dysfunction, adult respiratory distress syndrome, neurologic toxicity, renal and/or hepatic failure, and disseminated intravascular coagulation. Of particular concern is cardiac dysfunction, which can be rapid onset and severe, but is typically reversible. The pathophysiology of acute cardiac toxicity in the setting of CRS is not clear, but resembles cardiomyopathy associated with sepsis31 and stress cardiomyopathy, also called Takotsubo cardiomyopathy.32 Neurologic symptoms occurring in the context of CRS are varied (Table 1) and may occur coincident with other symptoms of CRS or may arise when the other symptoms of CRS are resolving (case 3). Magnetic resonance imaging often reveals no abnormalities, although in 1 case, we noted an abnormality in the splenium, consistent with mild encephalopathy with reversible splenial lesion syndrome (MERS), a syndrome associated with infection and reversible neurologic symptoms.33 Given the emerging understanding of a central role for IL-6 in CRS and the evidence that IL-6 can directly mediate neurotoxicity,34,35 it is of interest that MERS has also been reported to be associated with elevated IL-6 levels in the cerebrospinal fluid.36
CRS may also be associated with findings of macrophage activation syndrome/hemophagocytoic lymphohistiocytosis (HLH), and the physiology of the syndromes may have some overlap.37 Indeed, 1 patient who developed severe CRS associated with HLH following CD19-CAR therapy for ALL was found to carry a mutation in the perforin gene, which predisposes to HLH.8 This raises the prospect that host factors may play an important role in predisposing individuals to severe CRS in the setting of immune-based therapies, and more studies are needed to determine whether genetic predisposition contributes to this syndrome. Tumor lysis syndrome may also occur coincident with CRS, because massive immune cell activation and expansion correlates with antitumor efficacy. When this occurs, concomitant therapies for appropriate management of tumor lysis are essential for optimal clinical outcome.
Biomarkers
Because CRS occurs as a direct result of supraphysiological levels of inflammatory cytokines, it stands to reason that circulating cytokine levels could serve as biomarkers to diagnose and potentially quantify syndrome severity. However, several issues limit the effectiveness of this approach at the current time. First, using a biomarker as a basis for clinical management requires clinical laboratory improvement amendments (CLIA)-certified assays. Such assays are not readily available to measure cytokines in most hospitals at the current time. Second, although scientific and clinical evidence implicates a relationship between inflammatory cytokine levels and CRS severity,26 it remains unclear whether severity in an individual patient can be predicted based on cytokine levels. This is particularly challenging in patients with cancer, wherein baseline inflammatory cytokine levels can be very high due to their underlying disease. For this reason, fold increases, net increases, or rate of change in cytokine levels may provide better correlates of CRS severity than absolute cytokine levels.26 Third, diagnostic utility could require a profile of several different cytokines rather than changes in only 1 level. Finally, as discussed below, first-line immunosuppressive therapy for severe and life-threatening CRS is currently directed at preventing IL-6 signaling, despite the recognition that CRS is associated with elevations of several inflammatory cytokines. Although future studies may identify settings with greater or lesser dependence on the IL-6 axis, at the current time, based on clinical experience, we recommend use of tocilizumab as first-line immunosuppressive therapy, regardless of whether an individual patient demonstrates higher levels of TNFα or IL-1 compared with IL-6. Therefore, we conclude that real-time analysis of a broad panel of cytokines will not significantly impact management of individual patients with CRS at the current time and recommend that treatment decisions be based on clinical parameters.
CRP is an acute phase reactant produced by the liver largely in response to IL-6, and CRP levels serve as a reliable surrogate for IL-6 bioactivity.38,39 During CRS, CRP can increase by several logs, and this assay is rapid, inexpensive, and readily available in most hospitals. In some series, peak CRP levels and fold change in CRP have identified patients at risk for severe CRS.26 In addition, we found that declining CRP levels, although potentially lagging behind declines in cytokine levels by 1 to 2 days, may be useful to identify the peak of the syndrome in an individual patient. For these reasons, routine monitoring of CRP levels during the time of CRS symptomatology may have clinical utility. It is important to emphasize, however, that CRP levels are also elevated during infection and cannot be used to distinguish between infection-associated and noninfectious inflammation.40 We also noted extreme elevations of ferritin in many patients with CRS after CAR T-cell infusion, which further supports a link between CRS and macrophage activation syndrome/HLH, but in our experience, ferritin has not demonstrated utility in predicting CRS severity.
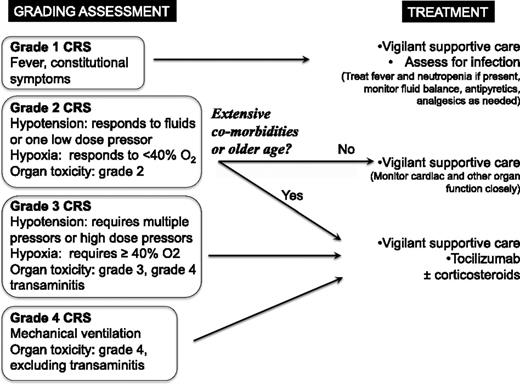
Grading system and treatment algorithm
The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE v4.0) contains a grading system that was designed for CRS associated with antibody therapeutics (supplemental Table 1, available on the Blood Web site). We modified this grading system to define mild, moderate, severe, and life-threatening CRS regardless of the inciting agent and to guide treatment recommendations (Table 2). Because many patients with CRS have overlapping symptomatology due to fever and neutropenia, infection, tumor lysis syndrome, or other medical complications, it is essential that attribution be carefully considered. This is particularly important given that the treatment algorithm relies heavily on the grading schema and incorporates immunosuppression. One could imagine, for example, a patient with hypotension related to sepsis wherein intervention with tocilizumab or corticosteroids (see below) would not be indicated. Hence, accurate application of this grading system requires clinical judgment to confirm that the symptoms are most likely due to CRS rather than another medical condition.
The clinical symptomatology comprising CRS is an indicator of response in the setting of immune-based therapies. It remains unclear to what extent the cytokines mediating the symptomology are required for antitumor effects. Hence, the goal of management is not to extinguish all evidence of CRS but to prevent life-threatening toxicity while maximizing the potential for antitumor effects. For this reason, we recommend symptomatic treatment of grade 1 CRS (Figure 2, case 1). We defined CRS as grade 2 when the patient develops hypotension responsive to fluids or 1 low-dose vasopressor or mild respiratory symptoms responsive to low flow oxygen (<40% FiO2) or grade 2 organ toxicity. Because hypotension is a major driver of severity grading, it is imperative that a clear baseline blood pressure be established prior to initiation of therapy that could induce CRS. As shown in Figure 2, the decision to intervene with immunosuppressive agents for patients with grade 2 CRS is influenced by the degree to which the patient is judged to be able to tolerate the altered hemodynamics and organ stresses associated with the syndrome. In older patients and patients with significant comorbidities, depending on clinical judgment, it may be appropriate to intervene with immunosuppression in patients with grade 2 CRS.
Treatment algorithm for management of CRS based on the revised CRS grading system. The algorithm uses the revised grading system for CRS to direct clinical management for patients with immunotherapy-associated CRS. We recommend vigilant supportive care including empiric treatment of concurrent bacterial infections and maintenance of adequate hydration and blood pressure for every grade. Immunosuppression should be used in all patients with grade 3 or 4 CRS and instituted earlier in patients with extensive comorbidities or older age. Grades 2-4 organ toxicities are dictated by CTCAE v4.0.
Treatment algorithm for management of CRS based on the revised CRS grading system. The algorithm uses the revised grading system for CRS to direct clinical management for patients with immunotherapy-associated CRS. We recommend vigilant supportive care including empiric treatment of concurrent bacterial infections and maintenance of adequate hydration and blood pressure for every grade. Immunosuppression should be used in all patients with grade 3 or 4 CRS and instituted earlier in patients with extensive comorbidities or older age. Grades 2-4 organ toxicities are dictated by CTCAE v4.0.
Patients in whom fluid therapy and 1 low-dose vasopressor are not sufficient to reverse hypotension are classified as severe or grade 3 CRS. Similarly, patients who require more than low flow oxygen or show evidence for grade 3 organ toxicity, including but not limited to coagulopathy, renal, or cardiac dysfunction, should be considered grade 3. Patients with grade 3 CRS need to be monitored very closely, likely in an intensive care unit with 1:1 nursing care. Importantly, in patients with grade 2 or grade 3 CRS, careful attention should be paid to cardiac function, as cardiac decompensation may occur and may not be readily evident without careful monitoring. Frequent echocardiographic monitoring may be indicated in patients in whom there is a concern of cardiac dysfunction. All patients with grade 3 CRS should be treated with immunosuppressive agents because of the risk for progression and the potential for irreversible organ dysfunction, with the goal of preventing progression to grade 4. Grade 4 CRS occurs when patients experience toxicity that is immediately life threatening, including a need for mechanical ventilation or grade 4 organ toxicity. We recommend that all patients with grade 4 CRS be treated with immunosuppressive agents in an attempt to suppress the inflammatory cascade and to prevent irreversible organ dysfunction.
Tocilizumab
Tocilizumab is a humanized, immunoglobulin G1κ (IgG1κ) anti-human IL-6R mAb approved for treatment of rheumatoid arthritis,41 juvenile idiopathic arthritis,42 and polyarticular juvenile rheumatoid arthritis. It has also been reported to have activity in Castleman disease,43 Crohn’s disease,44 and steroid refractory chronic graft-versus-host disease.45 Tocilizumab prevents IL-6 binding to both cell-associated and soluble IL-6Rs and therefore inhibits both classical and trans-IL-6 signaling (Figure 1). Emerging clinical experience at several institutions has concluded that tocilizumab is an effective treatment for severe or life-threatening CRS.8,22,24 The dose of tocilizumab approved for adults with rheumatoid arthritis is 4 to 8 mg/kg every 4 weeks, and the pediatric recommended dose is 8 to 12 mg/kg every 2 to 4 weeks. Using these recommendations as a guide, we typically administer tocilizumab over 1 hour at a dose of 4 mg/kg in adults and 8 mg/kg in children, with an option to repeat the dose if clinical improvement does not occur within 24 to 48 hours.
Side effects attributed to tocilizumab in rheumatologic disease, where the drug is given chronically, include transaminitis, thrombocytopenia, elevated cholesterol, and low-density lipoproteins.41 Neutropenia has also been uncommonly attributed to tocilizumab therapy, and this appears to resolve with discontinuation of the agent. The incidence of viral, bacterial, fungal, and mycobacterial infection is modestly increased in patients receiving chronic therapy for rheumatologic disease, thus providing the basis for a black box warning associated with the agent.41 We have not observed acute infusional toxicities secondary to tocilizumab in patients treated on our studies.
In patients with CRS who respond to tocilizumab, fever and hypotension often resolve within a few hours, and pressors and other supportive care measures can be weaned quickly thereafter. In some cases, however, symptoms may not completely resolve, and continued aggressive support may be necessary for several days (case 3). If the patient’s condition does not improve or stabilize within 24 hours of the tocilizumab dose, administration of a second dose of tocilizumab and/or a second immunosuppressive agent, such as corticosteroids, should be considered. Whether lack of response is related to ongoing production of IL-6, inadequate dosing of tocilizumab, or other factors is not yet known.
As discussed above, neurologic symptoms associated with CRS sometimes follow a different time course of onset and resolution (case 3). We have occasionally witnessed patients whose hemodynamic instability resolves rapidly following administration of tocilizumab, but who subsequently develop signs and symptoms of neurotoxicity. Based on the following evidence, we hypothesize that this relates to IL-6-directed neurotoxicity. First, IL-6 has been reported to mediate substantial neurologic effects and has been implicated in several neurologic conditions spanning Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, schizophrenia, depression, and MERS.34-36 Second, elevated central nervous system IL-6 levels are likely to occur in CRS due to direct transit of IL-6 via production in the periphery and/or production of IL-6 via trafficking of activated immune cells to the CNS. Indeed, we have observed elevated IL-6 levels in the cerebrospinal fluid associated with neurotoxicity. Third, IL-6 levels rise transiently following administration of tocilizumab due to blockade of the receptor,46 which inhibits receptor-mediated clearance, and tocilizumab is not expected to cross the blood:brain barrier. Hence, elevations in IL-6 within the CNS are not likely to be directly ameliorated in the short term and may even be transiently aggravated by tocilizumab therapy. For these reasons, for patients with grade 3 or 4 CRS associated with neurologic dysfunction without significant hemodynamic instability or other life-threatening symptomatology, consideration may be given to the use of corticosteroids as a preferred first-line immunosuppressive.
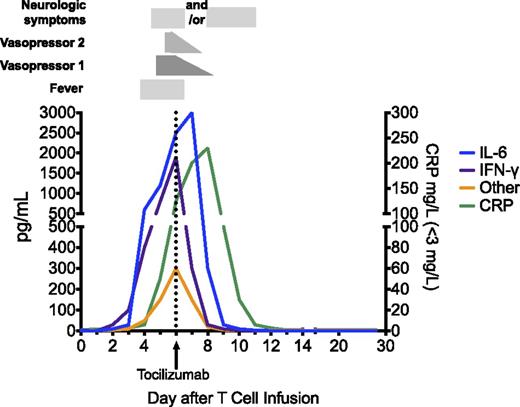
Figure 3 depicts a hypothetical patient with grade 3 CRS who develops fever on day 3, then hypotension on day 5 that is initially managed with a low-dose vasopressor, and then tocilizumab on day 6 after cardiovascular decompensation. IL-6 and IFNγ peak coincident with decompensation. IFNγ rapidly returns to baseline after tocilizumab, but owing to its mechanism of action, whereby receptor-mediated endocytosis of IL-6 is blocked by tocilizumab, IL-6 levels typically rise transiently immediately following tocilizumab before returning to baseline. Numerous other cytokines are also elevated and follow a similar time course. As depicted, neurologic symptoms can manifest either before or after tocilizumab administration and not infrequently persist for some time after tocilizumab, presumably due to its inability to cross the blood:brain barrier.
Cytokine changes associated with clinical findings in a hypothetical patient with grade 3 CRS. A dramatic rise in IL-6 and IFNγ levels is associated with the onset of fever at day 3 after CAR T-cell infusion. Despite vigilant supportive care, the patient becomes hypotensive, requiring the use of 1 vasopressor on day 5. After a brief period of cardiovascular stability, a second vasopressor is required to maintain adequate perfusion on day 6, at which time tocilizumab is administered. IL-6 levels continue to rise transiently after tocilizumab, because it continues to be produced and tocilizumab blocks IL-6R-mediated endocytosis. Vasopressor support is gradually weaned over the next 48 hours, although neurologic changes may persist or initially manifest after tocilizumab, but eventually resolve. Several other inflammatory cytokines, including TNFα, IL-2, GM-CSF, and others noted in the text are also likely to be elevated during the peak of the syndrome.
Cytokine changes associated with clinical findings in a hypothetical patient with grade 3 CRS. A dramatic rise in IL-6 and IFNγ levels is associated with the onset of fever at day 3 after CAR T-cell infusion. Despite vigilant supportive care, the patient becomes hypotensive, requiring the use of 1 vasopressor on day 5. After a brief period of cardiovascular stability, a second vasopressor is required to maintain adequate perfusion on day 6, at which time tocilizumab is administered. IL-6 levels continue to rise transiently after tocilizumab, because it continues to be produced and tocilizumab blocks IL-6R-mediated endocytosis. Vasopressor support is gradually weaned over the next 48 hours, although neurologic changes may persist or initially manifest after tocilizumab, but eventually resolve. Several other inflammatory cytokines, including TNFα, IL-2, GM-CSF, and others noted in the text are also likely to be elevated during the peak of the syndrome.
Corticosteroids and other agents
Clinical experience also demonstrates that corticosteroids are effective treatment of CRS, and a rapid steroid taper can generally be accomplished within several days without recurrence of the CRS. Despite this, we currently consider corticosteroids as second-line therapy for CRS. This is based on our clinical observation that response to tocilizumab may be more rapid than response to corticosteroids. In addition, corticosteroids appear to have more widespread effects on the immune system, and emerging evidence suggests that they may mediate a greater adverse effect on the antitumor activity of adoptively transferred T cells.26 We acknowledge, however, that the recommendation to use tocilizumab over corticosteroids is based on limited clinical experience and that in some settings wherein immune toxicity is associated with antitumor therapies, such as graft-versus-host disease and checkpoint inhibitor therapy, corticosteroids do not prevent therapeutic benefit.1,47 Thus, some physicians may choose to use corticosteroids as a frontline agent or to use both agents in cases of severe or life-threatening CRS. Although dosing and choice of corticosteroid should be tailored to the individual patient, commonly used initial doses include methylprednisolone (2 mg/kg/day), which can generally be weaned over several days. For patients with severe neurologic symptoms, consideration may be given to using dexamethasone (0.5 mg/kg; maximum, 10 mg/dose) due to more efficient penetration of the blood:brain barrier,48 although evidence for differential efficacy of methylprednisolone vs dexamethasone in this setting has not been established. It is also important to note that, especially in the case of B-ALL, many patients have chronically received corticosteroids as part of their treatment regimen, and therefore some patients who experience CRS may have a relative corticosteroid deficiency due to chronic suppression of their hypothalamic-pituitary axis wherein stress doses of hydrocortisone may be indicated.
Targeted immunosuppressive agents are also available to inhibit TNFα and IL-1, both of which may contribute to CRS. Hence, anti-TNFα mAbs (infliximab) and soluble TNFα receptor (etanercept) and IL-1R-based inhibitors (anakinra) could also provide benefit and have been used successfully (case 2). These agents have also demonstrated efficacy in the setting of macrophage activation syndrome and other syndromes49-51 that likely overlap with CRS in terms of pathophysiology. However, because supportive care with tocilizumab ± corticosteroids has been efficacious in our experience, we are not currently routinely using these agents.
Conclusion
Modern antitumor immunotherapies show impressive promise, but effective application of this new class of therapeutics requires that clinicians learn to recognize and manage novel toxicities associated with tumor immunotherapy. The overarching goal of CRS management in patients treated with immunotherapy for cancer is to prevent life-threatening CRS while maintaining the greatest chance for a beneficial antitumor effect. Here, we outlined a proactive management strategy that incorporates a grading system and treatment algorithm designed to administer early immunosuppression for patients at highest risk while avoiding unnecessary immunosuppression due to the potential risk of diminishing antitumor efficacy. Future work is needed to better understand the pathophysiology of this syndrome, to more definitely delineate the aspects of immune activation required for antitumor effects, and to validate optimal treatment strategies. Given the limited clinical experience with the syndrome, consideration could be given to a national or international registry to monitor outcomes and glean further insight into the spectrum of symptomatology and optimal management of CRS. Further, it is important to emphasize that the severity of CRS is greater in patients with higher disease burdens. Incorporation of immunotherapy into regimens that administer these therapies to patients with lower disease burdens would be expected to substantially reduce the toxicity observed and potentially benefit patients in the absence of any clinical evidence for CRS.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported, in part, by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. D.W.L. receives research support from the St. Baldrick’s Foundation, and S.A.G. receives research support from the Pennsylvania Department of Health. Research was also supported by a Stand Up To Cancer–St. Baldrick’s Pediatric Dream Team Translational Cancer Research Grant. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Authorship
Contributions: D.W.L., R.G., D.L.P., C.U.L., N.A., M.J., S.A.G., and C.L.M. wrote and edited the manuscript.
Conflict-of interest disclosure: M.J. has ownership interest in Juno therapeutics. D.L.P. has a patent in the field of adoptive immunotherapy and sponsored research support form Novartis. S.A.G. consults for and received research support from Novartis. C.L.M. has a patent in the field of adoptive immunotherapy and sponsored research support from Neomune. C.U.L. is a member of the Scientific Advisory Boards for TeamConnor, Solving Kid's Cancer, and Kids' Cancer Research Foundation. The Center for Cell and Gene Therapy has a collaborative research agreement with Celgene (N.A.). All other authors declare no competing financial interests.
Correspondence: Crystal L. Mackall, 10-CRC, 1W-3750, 10 Center Dr, MSC 1104, Pediatric Oncology Branch, National Cancer Institute, Bethesda, MD 20892; e-mail: mackallc@mail.nih.gov.