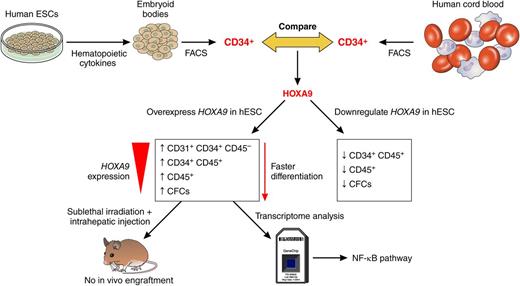
In this issue of Blood, Ramos-Mejía et al provide compelling evidence for HOXA9 as a key factor that can enhance and accelerate the differentiation of these cells to blood progenitor cells in vitro.1
Schematic representation of work undertaken by Ramos-Mejía et al to uncover the function of HOXA9 during hESC differentiation to blood cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
Schematic representation of work undertaken by Ramos-Mejía et al to uncover the function of HOXA9 during hESC differentiation to blood cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
Blood alone moves the wheels of history,” said Martin Luther in the 16th century. Blood—such a fragile and complex tissue, indispensable for normal physiology and often involved in human disease—has fascinated scientists, politicians, historians, filmmakers, and thriller book writers for centuries. A single adult contains approximately 3 × 1013 blood cells, millions of which must be replaced daily, however our understanding of the stem cells that are the ultimate source from which all blood cells arise is incomplete. Located deep within the bone marrow, hematopoietic stem cells (HSCs) are indispensable for the body’s homeostasis and yet they are few in number, with typically only 1 in every 10 000 cells having a recognizable stem-cell phenotype. Nevertheless, their very value to human health has made HSCs an attractive target for investigation. The fact that their use in the treatment of hematopoietic malignancies was one of the first successful stem-cell treatments is testament to this, but our options to obtain therapeutically useful HSCs are limited. Donated bone marrow and umbilical cord blood are in short supply and carry the risk of graft-versus-host disease and the likelihood of DNA damage caused by age and/or lifestyle; hence, the quest for new sources of blood stem cells has become a worldwide effort in the last decade. The most promising source seems to be pluripotent stem cells such as human embryonic stem cells (hESCs)2 and induced pluripotent stem cells (hiPSCs).3 The attraction lies in their apparently youthful and highly proliferative nature coupled to their pluripotent phenotype, which can be directed toward blood-cell lineages using developmental signaling cues and hematopoietic factors.4,5 But despite this, hESC- and hiPSC-derived hematopoietic progenitors retain mostly an embryonic and fetal gene expression profile and do not confer long-term functional engraftment upon transplantation into irradiated immunocompromised mice.6 By analogy, we could not expect them to engraft in human patients, which greatly reduces their therapeutic utility.
In this article, Ramos-Mejía et al1 took advantage of the identification of key transcription factors that show a different gene expression pattern between hESCs and cord blood–derived progenitors (see figure).7 The main hypothesis behind this approach is that manipulating the expression of such genes might push hESC-derived blood progenitor cells to reach the cellular maturity necessary for long-term engraftment. Moreover, Ramos-Mejía et al provide compelling evidence that manipulating the expression of a single gene may be sufficient to enhance hESC differentiation to hematopoietic lineages in vitro. As is shown in the figure, the authors focus on HOXA9, a key regulator of hematopoiesis and leukemogenesis, and show that this gene is expressed at lower levels in hESC-derived blood progenitors when compared with progenitors derived from cord blood. Both the overexpression and inhibition studies performed by Ramos-Mejía et al show that HOXA9 is indeed a key factor for enhancing and accelerating the differentiation of hESC-derived hemogenic progenitors (defined as CD31+CD34+CD45–) to blood cells. Furthermore, HOXA9 directs the commitment of hESC-derived blood progenitors to the granulocytic lineage (when overexpressed) and erythroid lineages when repressed, suggesting multiple roles for HOXA9 at various stages of differentiation that are likely to involve different downstream targets. Despite this, HOXA9 overexpression is not sufficient to induce long-term engraftment of immunocompromised mice, indicating that other factors such as ERG, RORA, SOX4, and MYB may be needed, as shown in a recent publication.8
The authors of this study took an elegant approach to identify key signaling pathways that could be activated downstream of HOXA9, and through a transcriptomics approach, they found that 8 of the 20 top targets that were activated in response to HOXA9 activation were known to be implicated in the NF-κB pathway, already shown to play an important role in HSC homeostasis and differentiation.9 Although the current manuscript does not go further into the ways that NF-κB may affect the differentiation process, it is our hope that the authors will look more closely at the mechanistic links between NF-κB and HOXA9 and possible ways to switch this pathway on and off at will, using small-molecule activators and inhibitors. This type of approach may offer more potential for developing novel ways to generate long-term engrafting HSCs as genetic manipulation of stem cells is not desirable for clinical applications because of an increased risk of tumorigenesis. As in all cell replacement therapies, safety remains a key and important consideration, and although the authors show no evidence of karyotypic abnormalities in human ESC overexpressing HOXA9, it would be useful to investigate the occurrence of genomic instabilities in the target-blood-cell progenitors derived from those. HOXA9 is involved in leukemogenesis and has been reported to act together with MEIS1 to induce acute myeloid leukemia.10 Although no enhanced proliferation or preleukemic features were observed as a result of HOXA9 overexpression, it would be useful to use the most up-to-date genomic technologies to study any cell cycle-, proliferation-, and karyotype-related abnormalities in hESC-derived blood cells. Hence, the quest for understanding more about blood cells and the necessary HOX code goes on!
Conflict-of-interest disclosure: The authors declare no competing financial interests.