Abstract
Massive hemorrhage is associated with coagulopathy and high mortality. The transfusion guidelines up to 2006 recommended that resuscitation of massive hemorrhage should occur in successive steps using crystalloids, colloids, and red blood cells (RBCs) in the early phase and plasma and platelets in the late phase. With the introduction of the cell-based model of hemostasis in the mid-1990s, our understanding of the hemostatic process and of coagulopathy has improved. This has contributed to a change in resuscitation strategy and transfusion therapy of massive hemorrhage along with an acceptance of the adequacy of whole blood hemostatic tests to monitor these patients. Thus, in 2005, a strategy aiming at avoiding coagulopathy by proactive resuscitation with blood products in a balanced ratio of RBC:plasma:platelets was introduced, and this has been reported to be associated with reduced mortality in observational studies. Concurrently, whole blood viscoelastic hemostatic assays have gained acceptance by allowing a rapid and timely identification of coagulopathy along with enabling an individualized, goal-directed transfusion therapy. These strategies joined together seem beneficial for patient outcome, although final evidence on outcome from randomized controlled trials are lacking. We present how we in Copenhagen and Houston, today, manage patients with massive hemorrhage.
Introduction
Death from injury has increased by 20% over the last decade and accounts for more death than malaria, tubercolosis, and HIV combined.1 Hemorrhage requiring massive transfusion secondary to trauma and major surgery remains a major cause of potentially preventable deaths, and development of coagulopathy further substantiallyincreases the mortality rates of hemorrhaging patients.2 Classically, massive transfusion has been defined as receiving >10 red blood cell (RBC) units in 24 hours, although recently, a change toward applying the rate of transfusion in a shorter time frame such as 2 or 6 hours has been broadly accepted.3,4 Historically, treatment of massive hemorrhage with blood products has been based on the “Berne” concept, dictating that resuscitation should occur in successive steps starting with RBCs and followed by plasma when ∼1 blood volume was substituted and including platelets when >2 blood volumes were substituted. This approach was incorporated into transfusion guidelines as exemplified by guidelines from the American Society of Anesthesiologists (ASA).5,6 Early in the new millennium, however, this transfusion paradigm was challenged mainly based on the results from the US Military in Iraq, where thawed AB fresh frozen plasma (FFP) was administered together with RBCs, as well as platelet concentrates (PCs) from the start of resuscitation.7 This resuscitation regimen was based on the notion that it was problematic to dilute the concentration of platelets and coagulation factors by RBCs before administering platelets and plasma to massively bleeding patients.8,9 Another concern regarding the transfusion guidelines was that conventional coagulation tests, like prothrombin time and partial thromboplastin time, were applied to identify patients in need of plasma substitution. However, the conventional plasma based tests only describe isolated fragments of the hemostatic process10,11 and are poorly associated with bleeding and transfusion requirements, as well as the platelet count itself does not reflect whether the platelets are hemostatically intact.12 The criticism of using the conventional coagulation tests were inspired by a new understanding of the hemostatic process, introduced by Hoffman and colleagues in the mid-1990s.13 A final major problem with the conventional coagulation analysis is that the time from blood sampling to availability of the results is too slow to be of clinical relevance in the massively bleeding patient.14
The hemostatic system and its monitoring
The classical cascade model of coagulation that was introduced >50 years ago15,16 was in 1994 replaced by the cell-based model of hemostasis, emphasizing the importance of tissue factor as the initiator of coagulation and the importance of cellular elements, ie, platelets, for intact hemostasis.17 Hemostasis occurs, according to the cell-based model, in 3 phases: initiation, amplification, and propagation, with the magnitude of the thrombin generation, the “thrombin burst,” ultimately determining the hemostatic capacity of the formed clot.18 Therefore, tests that reflect this new understanding of hemostasis such as the viscoelastical hemostatic assays (VHAs) thrombelastography (TEG) and rotational thrombelastometry (ROTEM) appear appropriate to accurately monitor hemostasis.19 VHAs assess the viscoelastic properties of coagulation in whole blood under low shear conditions (Figure 1). A high correlation exists between thrombin generation and thrombus formation as evaluated by VHA, and therefore, coagulopathies secondary to impaired thrombin generation are identified.20,21 Importantly, VHA can differentiate between low fibrinogen and reduced platelet function as the cause of impaired clot strength, as well as can identify systemic hyperfibrinolysis. The clinical value of VHA is corroborated by >30 clinical studies of patients with massive hemorrhage related to liver and cardiac surgery and trauma. These studies demonstrate superiority of VHA to monitor and guide hemostatic resuscitation compared with conventional coagulation tests, resulting in reduced transfusion requirements, need for re-do surgery, and mortality.22,23 Furthermore, The Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis recommended in 2013 the use of VHA-guided hemostatic therapy also in actively bleeding patients with hemophilia.24 It should be noted that the standard VHA tests, TEG and ROTEM, cannot measure pharmacological platelet inhibition and thus identify bleeding secondary to aspirin and/or adenosine 5′-diphosphate receptor inhibitors such as clopidogrel, effient, or ticagrelor.12 In these patients, the TEG Platelet Mapping Assay (Haemonetics Corp., Braintree, MA) or the whole blood platelet impedance aggregometry assay Multiplate (Roche, Basel, Switzerland) should be used, enabling identification of potential increased bleeding risk secondary to pharmacological platelet inhibition.
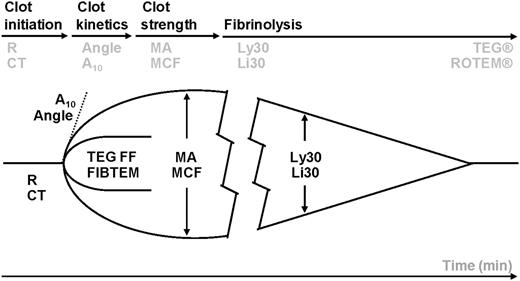
Whole blood is incubated at 37°C in a heated cup in which a suspended pin is connected to a detector system (a torsion wire in TEG and an optical detector in ROTEM). The cup and pin are oscillated relative to each other through an angle of 4° 45′. The movement is initiated from either the cup (TEG) or the pin (ROTEM). As fibrin strands forms between the cup and pin, the transmitted rotation from the cup to pin (TEG) or the impedance of the rotation of the pin (ROTEM) are detected at the pin, and a trace is generated. The trace is divided into parts that reflect different stages of the hemostatic process (clotting time, kinetics, strength, and lysis) with slightly different nomenclature for TEG and ROTEM.22 TEG and ROTEM parameters in the different phases of clot initiation, amplification, propagation, and degradation. TEG: R, reaction time (minutes); angle (degrees); MA, maximum amplitude (mm); Ly30, amplitude reduction after 30 minutes as an indicator of hyperfibrinolysis (%). ROTEM: CT, clotting time (seconds); A10, amplitude after 10 minutes (mm); MCF, maximum clot firmness (mm); Li30, amplitude reduction after 30 minutes as an indicator of hyperfibrinolysis (%). TEG functional fibrinogen maximal amplitude (mm). ROTEM FIBTEM (mm).
Whole blood is incubated at 37°C in a heated cup in which a suspended pin is connected to a detector system (a torsion wire in TEG and an optical detector in ROTEM). The cup and pin are oscillated relative to each other through an angle of 4° 45′. The movement is initiated from either the cup (TEG) or the pin (ROTEM). As fibrin strands forms between the cup and pin, the transmitted rotation from the cup to pin (TEG) or the impedance of the rotation of the pin (ROTEM) are detected at the pin, and a trace is generated. The trace is divided into parts that reflect different stages of the hemostatic process (clotting time, kinetics, strength, and lysis) with slightly different nomenclature for TEG and ROTEM.22 TEG and ROTEM parameters in the different phases of clot initiation, amplification, propagation, and degradation. TEG: R, reaction time (minutes); angle (degrees); MA, maximum amplitude (mm); Ly30, amplitude reduction after 30 minutes as an indicator of hyperfibrinolysis (%). ROTEM: CT, clotting time (seconds); A10, amplitude after 10 minutes (mm); MCF, maximum clot firmness (mm); Li30, amplitude reduction after 30 minutes as an indicator of hyperfibrinolysis (%). TEG functional fibrinogen maximal amplitude (mm). ROTEM FIBTEM (mm).
The Copenhagen concept
In 2005, we suggested that administration of plasma and platelets, together with RBCs, from the start of resuscitation would be beneficial in massively bleeding patients.8 We therefore introduced thawed plasma in our Blood Bank, enabling immediate delivery of transfusion packages (TPs) consisting of 5 prethawed AB RhD-negative single donor FFP, 2 O RhD-negative PCs (each consisting of pooled buffy coat platelets from 4 donors), and 5 leukoreduced O RhD-negative RBCs. This composition was based on a validation study of 10 patients presenting with uncontrolled hemorrhage receiving between 1 and 7 consecutive TPs.25 In these patients, hemostatic competence was evaluated by VHA before and after each administered TP, demonstrating that such an approach resulted in normal clot development and clot strength in all patients, including patients receiving >15 L of blood products. Furthermore, in a before-and-after study, we found improved 30-day survival in patients operated for a ruptured abdominal aortic aneurysm and receiving TPs during surgery (66% vs 44%, P = .02) compared with controls transfused according to the ASA guidelines.26
TPs are indicated in patients with uncontrolled hemorrhage, defined as loss of hemodynamic control and signs of hypoperfusion with need for blood product administration under pressure or through a rapid infusion system with a blood warmer to maintain an adequate mean arterial blood pressure. Simultaneously, the use of crystalloid fluids are paused, and colloids are not used. Typically, the causes are trauma, iatrogenic major vessel injury, obstetric calamities, or development of uncontrolled microvascular bleeding as judged by the attending physician. The TPs are administered at the clinician’s discretion and complemented by VHA analysis, which is repeated for every package administered, until surgical control has been established. Hereby, adjustments of the hemostatic resuscitation with adjusted FFP and PC doses, fibrinogen concentrate, cryoprecipitate, and/or tranexamic acid is instituted according to the patient’s VHA profile (Table 1; Figure 2). The aim of this resuscitation regimen is to maintain as normal a VHA profile as possible during the resuscitation phase, given that hypocoagulable VHA profiles have repeatedly been reported to be associated with increased mortality.14,27,28 Importantly, this strategy allows for an early shift toward hemostatic goal-directed therapy, guided by VHA algorithms (Table 1).
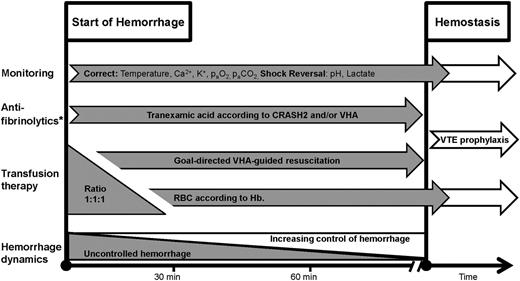
Hemostatic resuscitation as performed in Copenhagen, Denmark. The figure shows the phases from start of hemorrhage to control of bleeding (hemostasis). We initiate ratio 1:1:1 driven transfusion therapy of RBCs, FFP, and platelets during the initial phase of massive bleeding. VHA is performed on arrival, allowing for an early shift toward VHA-guided therapy subsequently. VHA is repeated during resuscitation. Simultaneously, tranexamic acid is administered according to the CRASH2 trial, and efforts are made to correct and reverse augmenting factors of coagulopathy and shock. *Antifibrinolytics may also be administered according to the VHA algorithm (Table 1). TXA, tranexamic acid; Hb, hemoglobin; VTE, venous thromboembolism.
Hemostatic resuscitation as performed in Copenhagen, Denmark. The figure shows the phases from start of hemorrhage to control of bleeding (hemostasis). We initiate ratio 1:1:1 driven transfusion therapy of RBCs, FFP, and platelets during the initial phase of massive bleeding. VHA is performed on arrival, allowing for an early shift toward VHA-guided therapy subsequently. VHA is repeated during resuscitation. Simultaneously, tranexamic acid is administered according to the CRASH2 trial, and efforts are made to correct and reverse augmenting factors of coagulopathy and shock. *Antifibrinolytics may also be administered according to the VHA algorithm (Table 1). TXA, tranexamic acid; Hb, hemoglobin; VTE, venous thromboembolism.
VHA is performed in the blood bank, with results displayed in real time at the bedside or in the operating room, trauma bay, and intensive care units. Furthermore, the time from arrival of the blood sample to the start of the VHA analysis (and hence access to the initial results) is <5 minutes.29 In surgical patients, it is the joint decision of the attending surgeon and the anesthetist to activate our massive transfusion protocol (MTP) encompassing the TP + VHA concept, whereas it is the trauma team leader that activates the MTP in trauma patients based on signs of hemorrhagic shock, injury pattern, and severity, as well as focused assessment with sonography for trauma severity. In trauma patients, blood samples for VHA and blood type and screen are obtained at hospital arrival so these patients only receive 1 TP with universal blood products, after which blood type-specific units are delivered. This is important given the limited availability of AB plasma.
The antifibrinolytic agent tranexamic acid, which acts by inhibiting activation of plasminogen to plasmin, is routinely administered at a dose of 20 mg/kg to patients with uncontrolled hemorrhage defined by the inability to maintain adequate blood pressure despite maximal infusion rate of blood products and/or microvascular bleeding as assessed by the surgeon. Also, patients with risk factors for hyperfibrinolysis presenting with uncontrolled bleeding such as obstetric calamities, reperfusion injury, and pelvic surgery routinely receive tranexamic acid. All trauma patients, however, are initially treated according to results from the Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage (CRASH2) trial, recommending tranexamic acid administration to bleeding trauma patients within 3 hours of the injury.30 Furthermore, during resuscitation in any massively bleeding patient including trauma, persistent hyperfibrinolysis as evaluated by kaolin activated TEG Ly30 >4%, together with diffuse, oozing bleeding, leads to (further) administration of tranexamic acid according to patient weight and bleeding dynamics. TEG Ly30 displays the percentage of the maximal clot strength that has been dissolved 30 minutes after maximum amplitude (MA) is reached.
Central to this concept is the close collaboration between the attending surgeon, the anesthetist, and the transfusion medicine expert in the blood bank, as the latter is automatically activated on delivery of TPs at any of our hospitals. The transfusion medicine expert ensures optimal logistical delivery of TPs, single blood products, and pro-hemostatics and guidance of the hemostatic resuscitation based on the clinical situation as described by the treating clinicians and the VHA results. It is pivotal that the MTP is discontinued in a timely manner, given the inherent risk of circulatory overload because rapid infusion systems are routinely used for patients with massive hemorrhage.
We conducted a before-and-after study in massively bleeding mixed surgical and trauma patients, of whom 390 patients were treated based on existing ASA 2006 guidelines and 442 patients received TPs and VHA-guided transfusion therapy.31 Those receiving the combination of TP and goal-directed VHA-guided resuscitation received more PCs and had a lower mortality compared with controls transfused in alignment with ASA 2006 guidelines (20% vs 32%, P = .02), suggesting that such a strategy may be beneficial for outcome.
In a study of 182 patients requiring full trauma team activation with an average injury severity score of 17 and 92% having a blunt trauma mechanism, this concept resulted in hemorrhage related mortality <15%.32 The average number of blood products transfused 2 hours after hospital arrival is 5 RBCs, 5 FFPs, and 2 PCs, equaling our TP, demonstrating our ability to administer balanced transfusion therapy from the start of resuscitation in patients with uncontrolled hemorrhage.32
Also, we routinely administer fibrinogen concentrate or cryoprecipitate to massively bleeding patients in a goal-directed way as evidenced by low TEG functional fibrinogen MA or profoundly increased TEG reaction time (R), respectively. We consider it vital to institute a balanced hemostatic resuscitation immediately after uncontrolled hemorrhage occurs or immediately on hospital admission because, regardless of how we choose to compose plasma, platelets, and RBCs in massively bleeding patients, the net hemostatic effect will be coagulopathic compared with the normal.8 Consequently, the longer delay in institution of balanced resuscitation, the more coagulopathic the final hemostatic competence will be. This concept of hemostatic resuscitation for patients with massive hemorrhage is implemented at all 10 hospitals in the Capital Region of Denmark that our Blood Bank serves, covering ∼2.5 million inhabitants.
For hospitals that do not have access to VHA, we recommend that a balanced transfusion therapy with blood products should be instituted aiming for a balanced FFP:PC:RBC ratio starting immediately when the massive bleeding is encountered and until surgical hemostasis is achieved. Furthermore, we recommend using tranexamic acid in patients with massive hemorrhage where increased fibrinolysis is prevalent, such as obstetric calamities, trauma, vascular surgery with reperfusion injuries, pelvic surgery, and intracranial hemorrhage.
The Houston concept
The concept we use in Houston is derived from the experience on the battlefield, supported by serial military and civilian studies identifying best practices.9,26,27,31-33 Before reaching the hospital, we liberally use tourniquets, hemostatic dressings, and hypotensive resuscitation in efforts to minimize and prevent ongoing blood loss.34 We have had plasma and RBCs on our 4 helicopters for the last 2 years, so patients in hemorrhagic shock are transfused with blood products in a balanced fashion before they reach the hospital.35 Recently, we switched to using liquid plasma as our plasma component before the patient reaches the hospital, because it has a longer shelf life and is similar in vitro hemostatic profile as thawed FFP.36,37 On arrival at our level 1 trauma center, TEG, hemoglobin, and a venous blood gas are immediately analyzed. In patients that have an assessment of blood consumption (ABC) score <2 (giving 1 point each for penetrating mechanism, systolic blood pressure <90 mmHg, heart rate >120 beats/minute, and a positive focused assessment with sonography for trauma or without substantial bleeding (<3 U/hour), we use the returning TEG laboratory values to drive resuscitation (Table 2).3,4,23,38 The use of Rapid TEG, which uses tissue factor in addition to kaolin as an activator and thereby produces the results faster than with kaolin alone, is based on an investigation of 1974 trauma patients having this analysis obtained at arrival. It was found that the Rapid TEG data were clinically superior to results from 5 conventional coagulation tests in identifying patients with an increased risk of early RBC, plasma, and platelet transfusions, as well as fibrinolysis.23 Moreover, Cotton and colleagues demonstrated that that Rapid TEG R results are available within minutes and are predictive of early transfusions of packed RBCs, plasma, and platelets.14 Patients that are in shock or hypotensive or have an ABC score39 ≥2 are started on our MTP protocol and receive 1:1:1 ratio -driven resuscitation. We have had plasma and RBCs in our emergency department for the last 4 years, so balanced transfusion is started immediately while waiting the average of 8 minutes it takes to receive 6 units of RBCs, 6 units of plasma, and 1 pack of apheresis platelets.40,41 Operative or interventional radiology is used quickly. Using data from our center, we found that trauma mortality increases when fibrinolysis increases to ≥3% as evidenced by TEG Ly30.42 Therefore, we infuse tranexamic acid according to the CRASH2 trial dose30 in patients that are bleeding, and Rapid TEG Ly30 ≥3% and are within a 3-hour window from injury. Response to resuscitation is closely tracked; as soon as bleeding slows, ratio-driven resuscitation converts to goal driven. We currently infuse very little crystalloid, which was the resuscitation fluid of choice until the recent concept of early administration of plasma, and very little if any artificial colloids.41 As with all trauma centers, damage control surgery is used when appropriate.43 Resuscitation after hemorrhagic injury is performed with blood products, and after hemostasis is obtained, low volume maintenance fluids with plasma lyte are used. We found that by avoiding an iatrogenic resuscitation injury and using hypertonic saline postoperatively, we can obtain midline fascial closure on >95% of patients.44 It is important to note that we do not exclusively use a ratio- or goal-driven approach. Rather we evaluate the individual patient, and if they are in shock or have a positive ABC score, we start with a ratio-driven approach, and when bleeding slows so that laboratory values will return in a clinically useful time period, we switch to a goal-directed approach. TEG values are used in a goal-directed fashion when the results can be used in a clinically relevant time frame.23
Discussion
The optimal way to resuscitate massively bleeding patients remains elusive, and adequately powered clinical trials addressing this topic are lacking. Instead, a large number of retrospective studies and before-and-after studies and systematic reviews concerning this topic have been published, although the interpretation of the currently available data differs substantially.45,46 In 2007, Borgman and colleagues reported their retrospective experiences in trauma patients receiving massive transfusion at an US Army combat support hospital in Iraq, reporting that a high ratio of FFP to RBCs was independently associated with improved survival, mainly by decreasing death from hemorrhage.47 This report was soon followed by several retrospective observational studies of patients (mainly trauma) receiving massive transfusion. The majority of these studies reported a significantly improved survival in patients receiving high ratios of FFP to RBCs compared with patients receiving low ratios.48 However, Snyder and colleagues demonstrated in 2009 that when including the time point of FFP transfusion in the resuscitation, the significant improvement in survival of high FFP:RBC ratios disappeared.49 A recent meta-analysis further concluded that survival bias and heterogeneity between studies preclude statistical comparisons concerning the effects of a 1:1 plasma to RBC transfusion ratio and that there was insufficient evidence to support a survival advantage with such a strategy.45,48 It is, however, undisputable that massively hemorrhaging patients who live long enough will receive more plasma than those who exsanguinate early.
Another point of controversy in regard to the ratio discussion is whether platelets should also be part of the immediate resuscitation. In 2008, Holcomb and colleagues reported on data from 466 massively transfused trauma patients and noted that those receiving high ratios of both FFP and PCs to RBCs had the highest survival, suggesting that massive transfusion guidelines should aim for a 1:1:1 FFP:PC:RBC ratio.50 Furthermore, Johansson and colleagues found in a multivariate logistic regression analysis that increasing the platelet count prior to transfusion was independently associated with a decreases in the odds ratio for mortality in massively bleeding patients.31 Also, Cotton and colleagues reported on the effect of implementation of TPs encompassing 10 RBCs, 4 FFPs, and 2 PCs, finding a significantly increased survival of massively transfused trauma patients receiving TPs compared with those treated before the implementation of this practice.51 Similarly, Gutierrez and colleagues demonstrated, in a retrospective study, that their MTP, enabling early delivery of plasma and platelets in addition to RBCs, resulted in favorable hematological indices in patients experiencing unanticipated or severe postpartum hemorrhage.52 We strongly agree that presently the optimal FFP:PC:RBC ratio remains elusive. However, it does appear that an early, more balanced, approach to transfusion appears to be associated with improved outcomes.40 Conversely, the Canadian Consensus conference in 2011 concluded that there was a lack of evidence in support of the 1:1:1 ratio.53
A much warranted multicenter randomized clinical study Pragmatic, Randomized Optimal Platelets and Plasma Ratios (clinicaltrials.gov identifier #NCT01545232) evaluating 2 different ratios of FFPs:PCs:RBCs (1:1:1 vs 1:1:2) in 680 trauma patients who were predicted to require massive transfusions finished enrollment December 2013, and the results of this study may guide the compositions of future MTP.54 It should be noted that using fixed ratios may increase the wastage of FFPs and PCs, although recently published data indicate that this problem can be adequately addressed.55 Conversely, most transfusion data suggest that early balanced transfusion decreases overall use of blood products while improving survival.56 Unquestionably, clear clinical goals for when to stop the MTP should be in place.57
When discussing different ratios of plasma, platelets, and RBCs for massively hemorrhaging patients, it should be emphasized that the timing of such therapy is pivotal for the success of the resuscitation. Riskin and colleagues reported a reduction in mortality from 45% to 19% after the implementation of an MTP. Interestingly, no change in the final calculated blood product ratios between before and after the implementation was found, but instead, they reported more expeditious product availability as a cause of the improved survival.58 This is in alignment with the findings of the Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) study reporting that early balanced plasma was associated with decreased 6-hour mortality, whereas this was not the case for receiving delayed but gradually balanced transfusion ratios.59,60 This notion was further corroborated by Rawdan and colleagues, who reported that moving thawed plasma from the blood bank to the emergency department, enabling early balanced blood product transfusion, was associated with a reduction in overall blood product use and a 60% decrease in odds of 30-day mortality.61 These studies all suggest that rapid infusion of balanced blood products into bleeding patients is critical to improved survival.
We recommend using VHA to monitor and goal direct hemostatic resuscitation in massively bleeding patients based on our published experience with this technology,26,27,31,32 and this is also recommended by American text books in transfusion medicine.62 Furthermore, it has been reported, especially from the trauma setting, that coagulopathy as evaluated by VHA is associated with increased transfusion requirements and mortality compared with noncoagulopathic patients.14,27,28,63 Additionally, a Cochrane review of 9 available randomized controlled trials, of which 8 were in cardiac surgery and 1 in orthotopic liver transplantation and consequently not in trauma patients, found a reduced bleeding and exposure to FFP and PC transfusion in patients treated according to VHAs compared with conventional analyses,64 and a later randomized controlled trial by Weber and colleagues corroborated these findings and further reported a reduced mortality.65 It could be argued that goal-directed hemostatic resuscitation may be superior to “blind” administration of TPs, but prospective, randomized clinical data are lacking that support either approach. We expect to initiate such a trial in 2015 involving 6 European level 1 equivalent trauma centers, funded by the European Union FP7 HEALTH INNOVATION PROGRAM entitled Targeted Action Curing Trauma Induced Coagulopathy.
Tranexamic acid has consistently been reported to reduce transfusion requirements in patients undergoing liver, cardiac, and orthopedic surgery, of which a subpopulation develops massive hemorrhage.66 The effect of tranexamic acid on mortality in patients undergoing emergency or urgent surgery, however, has not been established. The CRASH2 trial investigated the effect of tranexamic acid on blood transfusion and death in 20 127 trauma patients. Fifty percent did not bleed or need surgery.30 All-cause mortality was significantly reduced with tranexamic acid (14.5% vs 16.0%), as was the risk of death due to bleeding (4.9% vs 5.7%). This study has had significant impact on the European trauma centers that almost universally have adopted this regimen. In a follow-up exploratory analysis, it was reported that administration of tranexamic acid within 3 hours significantly reduced the risk of death due to bleeding, whereas treatment given after 3 hours seemed to increase the risk of death due to bleeding.67 We repeatedly identified hyperfibrinolysis by VHA in trauma patients with extensive tissue trauma receiving this therapy. Furthermore, patients with massive hemorrhage may have several blood volumes substituted, which may reduce the concentration of tranexamic acid to levels that may compromise its hemostatic effect.
Summary
Considering the high, and potentially preventable, mortality of massively bleeding patients, it is a prerequisite that a multidisciplinary team of hematologists, surgeons, emergency medicine, anesthetists, and blood bankers jointly develop and implement MTPs, together with clear indications for when this should be activated, stopped, and by whom. The MTP should encompass access to prethawed or liquid plasma, enabling immediate administration of a balanced transfusion of blood products, including plasma and platelets together with RBCs, after diagnosis of massive bleeding.
Given the current state of knowledge and the rapidity of changes in hemostatic disturbances in massively bleeding patients and that these patients may present several forms of coagulopathy, including hyperfibrinolysis, in Copenhagen, we recommend that whole blood VHAs are used to monitor and goal direct the hemostatic resuscitation with the aim of maintaining as normal a hemostatic capacity as possible throughout the resuscitation phase. Administration of antifibrinolytics should be given to bleeding trauma patients within 3 hours from injury.67 The effect of the antifibinolytic therapy should be monitored by VHA analysis because, in the most severely injured patients, additional administration of tranexamic acid is necessary to reverse this condition.
If VHA analysis is not available, a balanced transfusion therapy with blood products should be instituted, aiming for a balanced ratio starting immediately when the massive bleeding is encountered and until hemodynamic control is achieved.
In Houston, a somewhat different approach is taken, attempting to balance the benefits of both ratio- and goal-directed methods. We obtain initial VHA testing on all highest level activation patients and use the ABC scoring system to help identify and activate our MTP early. In bleeding patients, we start transfusing early with a balanced ratio (1:1:1) approach, and when bleeding slows enough where VHA results return to the bedside in a relevant time period, we switch to a goal-directed approach. Tranexamic acid is given to bleeding patients who also have excessive fibrinolysis (TEG Ly30 ≥ 3%). As outlined above, both these approaches have more similarities than differences and take into account local logistic challenges, the critical element of time, early use of blood product resuscitation, limitation of crystalloid and artificial colloids, and use of both ratio- and VHA-driven transfusion strategies.
Conclusions
Massive hemorrhage is a major cause of potentially preventable deaths, and development of coagulopathy further substantially increases the mortality rates of hemorrhaging patients.
Existing data indicate that immediate administration of a balanced transfusion therapy with plasma and platelets in addition to RBCs reduces mortality in patients with massive hemorrhage. After bleeding and transfusion has slowed so that VHA testing returns in a relevant time period, these whole blood assays may be used to monitor and guide hemostatic resuscitation of patients with massive hemorrhage. Timelines of an optimal transfusion strategy and VHA results are critical to improved survival in the massively bleeding patient.
Randomized controlled studies evaluating different hemostatic resuscitation regimens in patients with massive hemorrhage are highly warranted and are currently underway.54
Authorship
Contribution: P.I.J., S.R.O., R.O., J.S., C.E.W., and J.B.H. wrote and reviewed the manuscript, and reviewed and approved each other’s sections.
Conflict-of-interest disclosure: P.I.J. has received unrestricted research grants from Haemonetics Corp. and TEM International. C.E.W. has received unrestricted research grants from Haemonetic Corp. S.R.O., J.S, R.O., and J.B.H. declare no competing financial interests.
Correspondence: Pär I. Johansson, Capital Region Blood Bank, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; e-mail: per.johansson@rh.regionh.dk.