Key Points
Patients who relapse within 3 years of frontline FCR therapy have poor survival when treated with conventional salvage regimens.
Such patients are suitable for allogeneic stem cell transplantation and novel noncytotoxic therapies.
Abstract
Although fludarabine, cyclophosphamide, and rituximab (FCR) together are established as a standard first-line treatment of younger patients with chronic lymphocytic leukemia (CLL), there is little information to guide the management of patients with CLL refractory to, or who have relapsed after, receiving frontline FCR treatment. To define optimal salvage strategy and identify patients unsuitable for retreatment with FCR, we examined the survival and treatment outcome of 300 patients enrolled in a phase 2 study of FCR. After a median 142 months of follow-up, 156 patients developed progressive CLL, with a median survival of 51 months after disease progression. The duration of first remission (REM1) was a key determinant of survival after disease progression and first salvage. Patients with a short REM1 (<3 years) had a short survival period, irrespective of salvage therapy received; these patients have high unmet medical needs and are good candidates for investigation of novel therapies. In patients with a long REM1 (≥3 years), salvage treatment with either repeat FCR or lenalidomide-based therapy results in subsequent median survival exceeding 5 years; for these patients, FCR rechallenge represents a reasonable standard of care.
Introduction
Fludarabine, cyclophosphamide, and rituximab (FCR) together make up the most effective regimen in the treatment of patients with chronic lymphocytic leukemia (CLL).1-3 Despite the efficacy of FCR, the majority of patients are destined to relapse, and there is currently little data to guide the management of patients at the time of FCR failure. Chemotherapy with purine-analog–containing regimens induces a TP53-dependent cellular response4 and may contribute to the acquisition of (or selection for) a disease clone containing deletion and/or mutation of the TP53 gene.5 However, the acquisition of a TP53 abnormality may not be the only important consideration in patients in who FCR therapy fails; there may be other clinical and biological variables that influence the outcome of subsequent treatment.
To provide insight into the optimal management of patients who progress after frontline FCR treatment of CLL, we analyzed the long-term outcomes of patients treated in the MD Anderson phase 2 FCR study, last reported at a median of 6 years’ follow-up.2 The previously reported results were: response rate of 95%, complete response rate of 72%, and median time to disease progression of 80 months. The present analysis extends the median follow-up to 142 months and focuses on the outcomes of patients with refractory or relapsed disease.
Methods
FCR treatment and selection of study population
The treatment schedule for FCR was previously published.1,2 Between July 1999 and November 2003, 300 patients with previously untreated CLL and symptomatic disease were enrolled in an open-label, phase 2 evaluation of FCR as the initial therapy. Patients received rituximab (375-500 mg/m2) on day 1 and fludarabine (25-30 mg/m2 daily) and cyclophosphamide (250-300 mg/m2 daily) on days 1 to 3 of each course (days 2-4 for the first course only). Treatment was repeated every 4 weeks for a planned total of 6 courses. No maintenance treatment was given. This study was fully approved by the University of Texas MD Anderson Cancer Center’s institutional review board and was conducted in accordance with the Declaration of Helsinki.
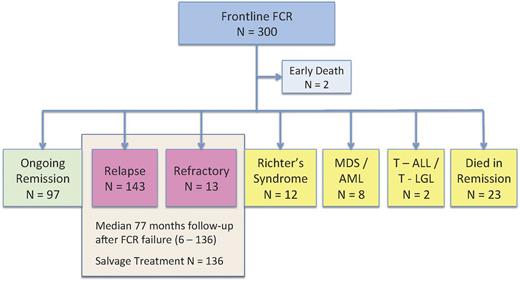
After a median follow-up time of 142 months (range 71-171), 168 patients had progressed disease: 143 with relapsed CLL, 13 with refractory CLL, and 12 with Richter transformation (Figure 1). The annual rate of relapse decreased over time, with the final progression occurring at 136 months and an apparent plateau on the relapse curve extending up to 171 months (supplemental Figure 1, available on the Blood Web site). Therefore, the majority of patients who were destined to relapse had already done so, permitting a mature analysis of postprogression outcomes. This study aimed to gain insight into the optimal management of patients with CLL progression after frontline treatment with FCR. Therefore, we restricted further prognostic factor and treatment analyses to the 156 patients without Richter transformation, of whom 136 (87%) had completed salvage therapy.
Flow diagram of 300 patients with CLL receiving frontline chemotherapy with FCR. The present analysis focuses on the outcome of 156 patients with relapsed (n = 143) or refractory (n = 13) disease. MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; T-ALL, T-cell acute lymphoblastic leukemia; T-LGL, T-cell large granular lymphocytic leukemia.
Flow diagram of 300 patients with CLL receiving frontline chemotherapy with FCR. The present analysis focuses on the outcome of 156 patients with relapsed (n = 143) or refractory (n = 13) disease. MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; T-ALL, T-cell acute lymphoblastic leukemia; T-LGL, T-cell large granular lymphocytic leukemia.
During the study period, there was no protocol-mandated salvage strategy for patients in whom disease had progressed after FCR, and patients were offered standard or investigational therapy at MD Anderson Cancer Center, or were treated locally, according to individual clinical status and physician discretion. To consolidate disparate salvage strategies, patients who received similar therapies were grouped together if the treatment outcomes of the individual salvage regimens were found to be similar on survival analysis.
Statistical considerations
First remission duration (REM1) was defined as the time period from the first date of FCR therapy to the date of disease progression. Survival analyses were performed from the date of disease progression for all patients with progressive CLL (n = 156) and were repeated (from the first date of salvage therapy) for the 136 patients who received salvage treatment. The purpose of the second analysis was to determine the impact of individual salvage therapies. All responses were defined according to the International Workshop on Chronic Lymphocytic Leukemia criteria.6 Continuous variables were evaluated using the Mann-Whitney U test, and categorical variables were evaluated using the Fisher exact or χ2 tests, as appropriate. The probabilities of overall survival (OS) were estimated using the Kaplan-Meier method,7 and comparisons were made using the log-rank test.8 Recursive partitioning and regression tree analyses9 were used to assist determination of optimal and clinically relevant cut points. Univariate and multiple Cox10 regression analyses were performed to assess the association between OS and patient characteristics. All P values were 2-sided and P values < .05 were deemed statistically significant.
Results
Survival after disease progression
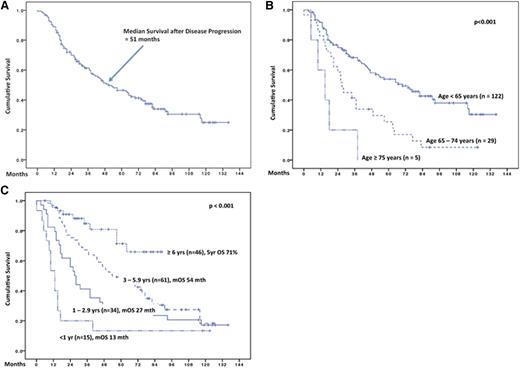
The median survival after disease progression was 51 months (Figure 2A). Of the 156 patients, 3 (2%) had active CLL but were too unwell to receive further treatment, 136 (87%) received salvage therapy at a median of 5 months after progression, and 16 (10%) had asymptomatic relapse and were treated using a “watch and wait” strategy; treatment status was unknown in 1 (<1%) patient. Supplemental Figure 2A shows similar postprogression survival for patients assigned to “watch and wait,” compared with those who received salvage therapy.
Survival of 156 patients after disease progression. The median follow-up period after progression was 77 months (range 6 - 136). (A) Survival of 156 patients after disease progression. The median follow-up period after progression was 77 months (range 6-136). (B) Survival after disease progression according to age at time of relapse/refractory disease. (C) Survival after disease progression according to duration of REM1 duration. Mth, months; yr, year; mOS, median overall survival.
Survival of 156 patients after disease progression. The median follow-up period after progression was 77 months (range 6 - 136). (A) Survival of 156 patients after disease progression. The median follow-up period after progression was 77 months (range 6-136). (B) Survival after disease progression according to age at time of relapse/refractory disease. (C) Survival after disease progression according to duration of REM1 duration. Mth, months; yr, year; mOS, median overall survival.
Factors significantly associated with inferior survival after disease progression were: older age, short REM1, and poor-risk cytogenetics at relapse (as determined by fluorescent in situ hybridization [FISH]) (Table 1). Patients aged <65, 65 to 74, and ≥75 years had median postprogression survivals of 70, 27, and 15 months, respectively (Figure 2B; P < .001). Regarding the impact of REM1 duration, there was a clustering of survival curves at REM1 of <1 year, 1 to 2.9 years, 3 to 5.9 years, and ≥6 years (Figure 2C and supplemental Figure 2B); median survivals for these 4 clusters were 13 months, 27 months, 54 months, and not reached (71% at 5 years), respectively (P < .001). Exploration of REM1 using recursive partitioning and regression tree analyses identified the best statistical cut-point to be 5.4 years (P = .00002). However, the group of patients with REM1 <5.4 years included some patients with relatively favorable survival (eg, median survival of 63 months for patients with REM1 of 4-4.9 years). Hence, we considered cut-points of 2 or 3 years to be more clinically relevant in identifying the poor risk population suitable for investigational therapy. Further comparisons of these 2 cut-points confirmed REM1 of < vs ≥3 years as being the better cut-point by P value, goodness-of-fit statistics (supplemental Table 1), and visual inspection (supplemental Figure 2B).
Characteristics and survival outcomes of 156 patients whose disease progressed after FCR therapy and 136 patients who subsequently received salvage therapy
| Characteristic . | At time of disease progression (N = 156) . | At time of first salvage treatment (N = 136) . | ||||
|---|---|---|---|---|---|---|
| n (%) . | Median survival (mo*) . | P . | n (%) . | Median survival (mo*) . | P . | |
| Age (y), median (range) | 56 y (17-85) | — | 56 y (17-76) | — | ||
| Age, y | ||||||
| <65 | 122 (78%) | 70 | <.001 | 109 (80%) | 63 | <.001 |
| 65-74 | 29 (19%) | 27 | 24 (18%) | 23 | ||
| ≥75 | 5 (3%) | 15 | 3 (2%) | 9 | ||
| Male | 117 (75%) | 51 | .54 | 103 (76%) | 42 | .53 |
| Female | 39 (25%) | 53 | 33 (24%) | 45 | ||
| First remission (REM1), y | ||||||
| <1 | 15 (10%) | 13 | <.001 | 10 (7%) | 9 | <.001 |
| 1-1.9 | 16 (10%) | 24 | 15 (11%) | 13 | ||
| 2-2.9 | 18 (12%) | 28 | 17 (13%) | 22 | ||
| 3-3.9 | 22 (14%) | 49 | 21 (15%) | 37 | ||
| 4-4.9 | 19 (12%) | 63 | 19 (14%) | 53 | ||
| 5-5.9 | 20 (13%) | 53 | 15 (11%) | 45 | ||
| ≥6 | 46 (29%) | 71% at 5 y | 39 (29%) | 64% at 5 y | ||
| Relapse Rai stage 0-2 | 104 (67%) | 54 | .41 | 89 (65%) | 43 | .50 |
| Relapse Rai stage 3-4 | 52 (33%) | 47 | 47 (35%) | 45 | ||
| Unmutated IgHV gene | 102 (79%) | 43 | .17 | 92 (79%) | 40 | .39 |
| Mutated IgHV gene | 27 (21%) | 77 | 25 (21%) | 74 | ||
| Unknown in 27 | Unknown in 19 | |||||
| Progression FISH (hierarchical) | ||||||
| 17p deletion | 21 (21%) | 33 | .04 | 21 (22%) | 29 | .10 |
| 11q deletion | 40 (40%) | 51 | 37 (39%) | 42 | ||
| Trisomy 12 | 9 (9%) | 77 | 9 (10%) | 63 | ||
| Negative panel | 17 (17%) | 63% at 5 y | 15 (16%) | 56% at 5 y | ||
| 13q deletion | 13 (13%) | 82 | 11 (12%) | 82 | ||
| Unknown in 56 | Unknown in 43 | |||||
| Salvage hemoglobin <0 g/dL | — | — | — | 23 (19%) | 22 | .22 |
| Salvage hemoglobin ≥10 g/dL | — | — | 101 (81%) | 45 | ||
| Unknown in 12 | ||||||
| Salvage WCC <100 × 109/L | — | — | — | 102 (82%) | 47 | .71 |
| Salvage WCC ≥100 × 109/L | — | — | 22 (18%) | 38 | ||
| Unknown in 12 | ||||||
| Salvage PLT <50 × 109/L | — | — | — | 15 (12%) | 13 | <.001 |
| Salvage PLT 50-99 × 109/L | — | — | 37 (30%) | 55 | ||
| Salvage PLT ≥100 × 109/L | — | — | 72 (58%) | 63 | ||
| Unknown in 12 | ||||||
| Salvage β2m <2 × ULN | — | — | — | 57 (53%) | 63 | .009 |
| Salvage β2m ≥2 × ULN | — | — | 50 (47%) | 37 | ||
| Unknown in 29 | ||||||
| Salvage regimen | ||||||
| FCR-based | — | — | — | 60 (44%) | 66 | .013 |
| Rituximab-based | — | — | 31 (23%) | 36 | ||
| Alemtuzumab-based | — | — | 15 (11%) | 28 | ||
| Intensive chemotherapy | — | — | 8 (6%) | 8 | ||
| Lenalidomide-based | — | — | 12 (9%) | 63 | ||
| Other regimens | — | — | 10 (7%) | 37 | ||
| Characteristic . | At time of disease progression (N = 156) . | At time of first salvage treatment (N = 136) . | ||||
|---|---|---|---|---|---|---|
| n (%) . | Median survival (mo*) . | P . | n (%) . | Median survival (mo*) . | P . | |
| Age (y), median (range) | 56 y (17-85) | — | 56 y (17-76) | — | ||
| Age, y | ||||||
| <65 | 122 (78%) | 70 | <.001 | 109 (80%) | 63 | <.001 |
| 65-74 | 29 (19%) | 27 | 24 (18%) | 23 | ||
| ≥75 | 5 (3%) | 15 | 3 (2%) | 9 | ||
| Male | 117 (75%) | 51 | .54 | 103 (76%) | 42 | .53 |
| Female | 39 (25%) | 53 | 33 (24%) | 45 | ||
| First remission (REM1), y | ||||||
| <1 | 15 (10%) | 13 | <.001 | 10 (7%) | 9 | <.001 |
| 1-1.9 | 16 (10%) | 24 | 15 (11%) | 13 | ||
| 2-2.9 | 18 (12%) | 28 | 17 (13%) | 22 | ||
| 3-3.9 | 22 (14%) | 49 | 21 (15%) | 37 | ||
| 4-4.9 | 19 (12%) | 63 | 19 (14%) | 53 | ||
| 5-5.9 | 20 (13%) | 53 | 15 (11%) | 45 | ||
| ≥6 | 46 (29%) | 71% at 5 y | 39 (29%) | 64% at 5 y | ||
| Relapse Rai stage 0-2 | 104 (67%) | 54 | .41 | 89 (65%) | 43 | .50 |
| Relapse Rai stage 3-4 | 52 (33%) | 47 | 47 (35%) | 45 | ||
| Unmutated IgHV gene | 102 (79%) | 43 | .17 | 92 (79%) | 40 | .39 |
| Mutated IgHV gene | 27 (21%) | 77 | 25 (21%) | 74 | ||
| Unknown in 27 | Unknown in 19 | |||||
| Progression FISH (hierarchical) | ||||||
| 17p deletion | 21 (21%) | 33 | .04 | 21 (22%) | 29 | .10 |
| 11q deletion | 40 (40%) | 51 | 37 (39%) | 42 | ||
| Trisomy 12 | 9 (9%) | 77 | 9 (10%) | 63 | ||
| Negative panel | 17 (17%) | 63% at 5 y | 15 (16%) | 56% at 5 y | ||
| 13q deletion | 13 (13%) | 82 | 11 (12%) | 82 | ||
| Unknown in 56 | Unknown in 43 | |||||
| Salvage hemoglobin <0 g/dL | — | — | — | 23 (19%) | 22 | .22 |
| Salvage hemoglobin ≥10 g/dL | — | — | 101 (81%) | 45 | ||
| Unknown in 12 | ||||||
| Salvage WCC <100 × 109/L | — | — | — | 102 (82%) | 47 | .71 |
| Salvage WCC ≥100 × 109/L | — | — | 22 (18%) | 38 | ||
| Unknown in 12 | ||||||
| Salvage PLT <50 × 109/L | — | — | — | 15 (12%) | 13 | <.001 |
| Salvage PLT 50-99 × 109/L | — | — | 37 (30%) | 55 | ||
| Salvage PLT ≥100 × 109/L | — | — | 72 (58%) | 63 | ||
| Unknown in 12 | ||||||
| Salvage β2m <2 × ULN | — | — | — | 57 (53%) | 63 | .009 |
| Salvage β2m ≥2 × ULN | — | — | 50 (47%) | 37 | ||
| Unknown in 29 | ||||||
| Salvage regimen | ||||||
| FCR-based | — | — | — | 60 (44%) | 66 | .013 |
| Rituximab-based | — | — | 31 (23%) | 36 | ||
| Alemtuzumab-based | — | — | 15 (11%) | 28 | ||
| Intensive chemotherapy | — | — | 8 (6%) | 8 | ||
| Lenalidomide-based | — | — | 12 (9%) | 63 | ||
| Other regimens | — | — | 10 (7%) | 37 | ||
WCC, white cell count; PLT, platelets; β2m, β2-microglobulin; ULN, upper limit of normal.
Unless otherwise stated.
According to FISH status at progression, patients with deletions of 17p and 11q experienced inferior postprogression survival compared with other cytogenetic subgroups (Table 1 and supplemental Figure 2C; P = .04). Multivariate analysis considering age, REM1, and 17p– status confirmed independent significance for all 3 factors in determining postprogression survival, with the strongest hazard ratio (HR) being assigned to REM1 of <3 years and 17p– status (Table 2).
Multivariate Cox proportional hazard models of survival
| Characteristic . | Survival from disease progression . | Survival from salvage therapy . | ||||
|---|---|---|---|---|---|---|
| N . | Hazard ratio (95% CI) . | P . | N . | Hazard ratio (95% CI) . | P . | |
| Age 65-74 y* | 156 | 2.1 (1.3-3.4) | .002 | 136 | 2.0 (1.2-3.3) | .009 |
| Age ≥75 y* | 5.7 (2.2-14.6) | <.001 | 4.8 (1.5-15.8) | .009 | ||
| REM1 <3 y vs ≥3 y | 2.2 (1.5-3.4) | <.001 | 2.1 (1.3-3.3) | .002 | ||
| Age 65-74 y* | 100 | 2.4 (1.3-4.5) | .006 | 93 | 2.6 (1.3-5.0) | .006 |
| REM1 <3 y vs ≥3 y | 3.5 (1.9-6.4) | <.001 | 3.6 (1.9-6.7) | <.001 | ||
| FISH (17p–)† | 3.5 (1.6-7.6) | .001 | 3.2 (1.5-7.0) | .003 | ||
| Age 65-74 y* | — | — | — | 124 | 1.8 (1.1-3.1) | .04 |
| Age ≥75 y* | — | — | — | 4.5 (1.1-18.9) | .04 | |
| REM1 <3 y vs ≥3 y | — | — | — | 1.7 (1.1-2.9) | .04 | |
| Salvage PLT <50 × 109/L | 4.4 (2.1-9.1) | <.001 | ||||
| REM1 <3 y vs ≥3 y | — | — | — | 77 | 2.4 (1.2-5.0) | .02 |
| FISH (17p–)† | — | — | — | 3.5 (1.4-9.1) | .008 | |
| Salvage β2m ≥2 × ULN | — | — | — | 2.6 (1.4-5.1) | .004 | |
| FCR or lenalidomide (vs rest) | — | — | — | 0.31 (0.15-0.64) | .001 | |
| Characteristic . | Survival from disease progression . | Survival from salvage therapy . | ||||
|---|---|---|---|---|---|---|
| N . | Hazard ratio (95% CI) . | P . | N . | Hazard ratio (95% CI) . | P . | |
| Age 65-74 y* | 156 | 2.1 (1.3-3.4) | .002 | 136 | 2.0 (1.2-3.3) | .009 |
| Age ≥75 y* | 5.7 (2.2-14.6) | <.001 | 4.8 (1.5-15.8) | .009 | ||
| REM1 <3 y vs ≥3 y | 2.2 (1.5-3.4) | <.001 | 2.1 (1.3-3.3) | .002 | ||
| Age 65-74 y* | 100 | 2.4 (1.3-4.5) | .006 | 93 | 2.6 (1.3-5.0) | .006 |
| REM1 <3 y vs ≥3 y | 3.5 (1.9-6.4) | <.001 | 3.6 (1.9-6.7) | <.001 | ||
| FISH (17p–)† | 3.5 (1.6-7.6) | .001 | 3.2 (1.5-7.0) | .003 | ||
| Age 65-74 y* | — | — | — | 124 | 1.8 (1.1-3.1) | .04 |
| Age ≥75 y* | — | — | — | 4.5 (1.1-18.9) | .04 | |
| REM1 <3 y vs ≥3 y | — | — | — | 1.7 (1.1-2.9) | .04 | |
| Salvage PLT <50 × 109/L | 4.4 (2.1-9.1) | <.001 | ||||
| REM1 <3 y vs ≥3 y | — | — | — | 77 | 2.4 (1.2-5.0) | .02 |
| FISH (17p–)† | — | — | — | 3.5 (1.4-9.1) | .008 | |
| Salvage β2m ≥2 × ULN | — | — | — | 2.6 (1.4-5.1) | .004 | |
| FCR or lenalidomide (vs rest) | — | — | — | 0.31 (0.15-0.64) | .001 | |
95% CI, 95% confidence interval.
Compared with age <65 years.
Compared with non-17p–, non-11q– cases.
Survival after salvage therapy
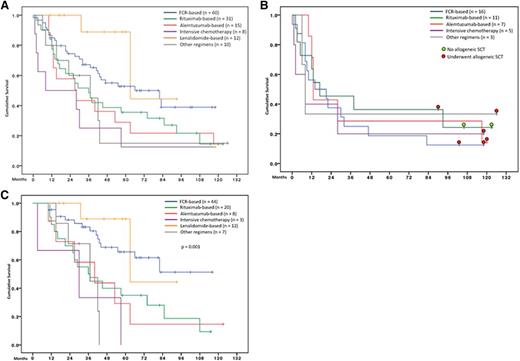
The median follow-up time of 136 patients who underwent salvage treatment was 63 months (range 11-126), and the median survival after salvage therapy was 43 months (supplemental Figure 3A). Factors significantly associated with inferior survival after salvage therapy were: older age, short REM1, platelets <50 × 109/L, high β2-microglobulin, and salvage regimen (Table 1, Figure 3A, and supplemental Figure 3D-E).
Survival after salvage therapy. (A) Survival after salvage therapy according to regimen received (n = 136; P = .013). (B) Survival after salvage therapy according to regimen received, in patients with first remission of <3 years (n = 42, P = .93). Colored dots represent transplantation status of survivors. (C) Survival after salvage therapy according to the regimen received in patients with remission of ≥3 years (n = 94). FCR or lenalidomide-based regimens were associated with improved survival (P < .001 vs rest).
Survival after salvage therapy. (A) Survival after salvage therapy according to regimen received (n = 136; P = .013). (B) Survival after salvage therapy according to regimen received, in patients with first remission of <3 years (n = 42, P = .93). Colored dots represent transplantation status of survivors. (C) Survival after salvage therapy according to the regimen received in patients with remission of ≥3 years (n = 94). FCR or lenalidomide-based regimens were associated with improved survival (P < .001 vs rest).
Analysis of REM1 in relation to postsalvage survival showed similar clustering of survival to that of postprogression survival (supplemental Figure 3B). Thus, patients with REM1 <3 years had a median postsalvage survival of 13 months compared with 63 months for patients with REM1 ≥3 years (P < .001). Because the dates of salvage therapy spanned a period of 12 years, we next sought to determine whether the apparent improved survival in patients with long remissions was a result of improvement in salvage technologies over the study period (referred to as “era effect”). This analysis (supplemental Figure 3C) confirmed that the prognostic significance of REM1 was not the result of an era effect.
Multivariate modeling of postsalvage survival showed independent significance for age, REM1, 17p– status, platelet count, β2-microglobulin, and salvage regimen (Table 2). Because the choice of salvage therapy is of particular clinical relevance, we further examined the effect of individual salvage strategies on survival.
Salvage strategy and effect on survival
We grouped salvage therapies into 6 categories with similar drug combinations and survival: FCR-based (n = 60), rituximab-based (n = 31), alemtuzumab-based (n = 15), lenalidomide-based (n = 8), intensive chemotherapy (n = 12), and other therapies (n = 10) (supplemental Table 2). Figure 3A shows postsalvage survival for the 6 categories. Although the choice of salvage regimen was highly individualized, we nevertheless noted the superior survival for patients receiving either FCR-based or lenalidmide-based salvage (median survival 82 months) compared with other categories (median survival 29 months; P < .001).
Next we sought to determine whether the effect of different salvage categories on survival was dependent on duration of REM1 (Figures 3B-C). For patients with short REM1 (<3 years), postsalvage survival was poor irrespective of the salvage strategy used (Figure 3B, median survival 8-17 months across different categories). This therapy resistance was not adequately explained by genomic features, because only 6 of 20 (30%) patients with REM1 <3 years carried deletion of 17p. Thus, initial FCR resistance itself is a bona fide predictive factor for poor response to subsequent salvage. Of interest, we note that 6 of 8 (75%) long-term survivors in the REM1 <3 year category had undergone allogeneic stem cell transplantation (Figure 3B). The timing of transplantation in patients with REM1 <3 years was heterogeneous and precluded derivation of further conclusions: among 12 patients, one received the transplant in second remission, four received the transplant after failing to achieve a second remission, and the remaining seven were transplanted beyond second relapse.
For patients with REM1 ≥3 years, survival was longest in patients who received FCR-based or lenalidomide-based salvage (median survival not reached, with 5-year survival of 70%) compared with other strategies (median survival 37 months; P < .001) (Figure 3C). Among these patients, the overall and complete response rates after rechallenge with FCR was 86% and 18%, respectively (supplemental Table 2). Of note, the patients in this report were largely treated before the advent of highly effective novel agents, with only 1 patient each receiving ABT-263 or ibrutinib as first-salvage therapy.
Discussion
A major limitation of this study is that patients were not treated uniformly on specified protocols post-FCR failure but were treated based on individual circumstances and physician discretion. Although this type of analysis is prone to bias and cannot account for heterogeneity in patient factors that may have influenced the choice of salvage therapy, the results are nevertheless valuable because they provide an insight into the “real-world” outcomes of patients treated in the academic and community settings. The unique feature of this data set is that the follow-up is very mature, capturing virtually all of the patients who were destined to relapse. This maturity is important because the biology of patients who relapse is not uniform over time: patients with high-risk disease relapse earlier than those with low-risk disease, and any premature analysis of salvage results will therefore be over-represented by patients with adverse biology, diluting the effectiveness of conventional interventions such as retreatment with FCR. In fact, our analysis established repeat FCR therapy as being highly effective in patients with a durable first remission, and FCR rechallenge is thus a reasonable standard of care for these patients.
A second major conclusion is that patients in whom FCR failed within 3 years should not be rechallenged, and in our experience their adverse prognosis is not rescued by alternative therapies such as alemtuzumab. These patients constitute a group with high unmet medical need. Indeed, the European Society for Blood and Marrow Transplantation, European Society for Medical Oncology, and the German CLL Study Group all recognize patients with early failure (defined empirically by these groups as <2 years) after fludarabine combination chemotherapy as being “ultra–high-risk.”11-13 Our experience showed that patients who progress between 2 and 3 years after FCR have an adverse survival similar to those who progress before 2 years, and detailed comparison of different cut-points identified 3 years as the most clinically relevant discriminator. This may be caused by the greater potency of FCR over previous fludarabine combinations,3 and thus a longer REM1 duration must be allowed to identify the same group of poor-risk patients. Our data indicate that the ultra–high-risk category should be extended to include treatment failure between 24 and 36 months in the FCR era.
Significant therapeutic advances have occurred since the patients in this study received their first salvage regimen. Tyrosine kinase inhibitors such as ibrutinib14 and idelalisib15 showed impressive activity in patients with heavily pretreated, genetically high-risk CLL, and ibrutinib is now licensed in the United States. Other promising modalities include novel anti-CD20 antibodies,16 bcl2 inhibitors,17,18 and chimeric antigen receptor T cells.19 These agents all show promising activity in CLL but are associated with considerable costs and are not affordable in many health care systems if applied broadly across large numbers of patients with CLL. To responsibly and effectively advance the development of these promising new therapies, they should be targeted specifically to groups in which they can provide the greatest benefit, such as those not suitable for FCR retreatment. Our experience and that of others11-13 suggest that an expanded ultra–high-risk category including (1) patients with TP53 aberrations and (2) those with early (<3 years) FCR failure represents an effective and practical way to identify such patients. Finally, patients with significant marrow failure and thrombocytopenia had very poor treatment outcomes in our series, largely because of poor tolerance of chemotherapy and exclusion from therapeutic trials. The nonmyelosuppressive nature of many of the novel agents provides an opportunity to offer these patients effective salvage treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.S.T., S.L., M.J.K., and W.P. analyzed the data and wrote the paper; S.O., W.W., A.F., J.C., I.K., and H.K. provided clinical care, contributed clinical observations, and reviewed the paper; and X.W. and K.-A.D. assisted in statistical analysis and reviewed the paper.
Conflict-of-interest disclosure: C.S.T. receives honorarium and research funding from Roche. The remaining authors declare no competing financial interests.
Susan Lerner died on October 22, 2013.
Correspondence: Michael J. Keating, Leukemia Department, Unit 428, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: mkeating@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal