Abstract
Background: Most acute myeloid leukemia (AML) patients (pts) stilldo not achieve long-term survival. Better risk stratification and novel therapeutic avenues are needed to improve pts outcomes. The expression of microRNAs has been demonstrated to be altered in AML & miR-based therapies are entering clinical trials. MiR-320a, maps to chromosome 8p21.3 & is known to play a role in several tumors; e.g. higher miR-320a expression suppresses the progression of colorectal cancer. In AML miR-320a has been shown to inhibit cell proliferation, likely by targeting the transferrin receptor 1.
Objective: The objective of this study was to investigate whether a differential expression of pri-miR-320a associated with outcome in AML pts.
Methods: We assessed the expression levels of pri-miR-320a, a precursor molecule of mature miR-320a. The pri-miR-320a expression levels in 129 AML pts were assessed by quantitative reverse transcription polymerase chain reaction & normalized to a housekeeping gene (18S). The 75th percentile was chosen as a cut-off discriminating between high & low pri-miR-320a expressers.
We analyzed 129 AML pts (median age at HCT 64 years [y]; range 22–74 y) who received reduced intensity conditioning (RIC; Fludarabine 30mg/m^2 at day -4 to -2 & 2 Gy total body irradiation at day 0)-hematopoietic cell transplantation (HCT) at the University of Leipzig, with pretreatment bone marrow available. The median follow-up was 4.5 y for pts alive. European LeukemiaNet (ELN) genetic classification was: favorable (n=33; 25.6%), intermediate I (n=33; 25.6%), intermediate II (n=29; 22.5%) or adverse (n=30; 23.3%). The pts were also characterized for FLT3-ITD status, CEBPA, IDH1, IDH2 and NPM1 mutations.
Results: At diagnosis high pri-miR-320a expression associated by trend with lower hemoglobin levels (P=.094), lower white blood cell counts (P=.079) & lower peripheral blast counts (P=.096). High pri-miR-320a expressers less frequently had NPM1 (P=.038) or CEBPA (P=.025) mutations. Interestingly, pri-miR-320a expression was significantly lower in pts with trisomy 8 (P=.018) compared to non-trisomy 8 pts & in the ELN intermediate II group none of the trisomy 8 pts was in the high pri-miR-320a expressing group.
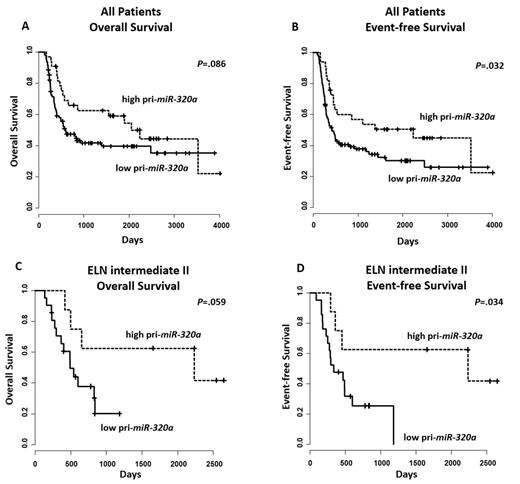
Analysis of our AML pts, showed a significant association between pri-miR-320a expression status & clinical outcome. In the entire group of pts high pri-miR-320a expressers had a longer overall survival (OS; P=.086; Figure 1) by trend & a significantly longer event-free survival (EFS; P=.032). The strongest impact of pri-miR-320a was found in the ELN intermediate II group, where high pri-miR-320a expression was associated by trend with longer OS (P=.059; Figure 1) & a significantly longer EFS (P=.034).
In multivariate analysis, the prognostic impact of pri-miR-320a expression status was confirmed in the entire group of pts (OS: P<.01, hazard ratio (HR) 0.45, 95% confidence interval (CI) 0.27-0.75; EFS: P<.01, HR 0.45, 95% CI 0.28-0.71).
Conclusion: High expression of pri-miR-320a associates with distinct clinical & molecular features. Interestingly, in AML pts with trisomy 8 pri-miR-320a that maps to chromosome 8 has a significantly lower expression, indicating a possible leukemogenic link to trisomy 8 associated AML. High pri-miR-320a expression significantly associates with better outcome especially in the ELN intermediate II group. These results suggest that pri-miR-320a could be used as a marker to refine current AML risk stratifications. Increasing miR-320a, by e.g. miR-replacement therapies, might improve outcomes of AML pts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.