Abstract
Hemophilia A is an X-linked disorder characterized by a deficiency or absence of blood coagulation protein factor VIII (fVIII). Treatment involves replacement of fVIII through infusions for acute bleeding episodes or prevention of bleeding events. Approximately 30% of individuals with severe hemophilia A will develop antibodies to fVIII. Many studies have characterized the antigenic properties of the C2 and A2 domains as these domains are considered the predominant immunogenic domains of the fVIII protein. However, there is increasing evidence that the C1 domain contributes to fVIII function and immune response to fVIII.
Our laboratory has produced and purified a murine IgG2ak anti-human C1 domain monoclonal antibody (MAb), designated 2A9. In this study, we characterized the functional properties of MAb 2A9 using standard coagulation testing including its anti-fVIII inhibitor titer by Bethesda assay and its ability to inhibit fVIII binding to von Willebrand factor (VWF) by competition ELISA. In the Bethesda assay, 2A9 has an inhibitor titer of 97 BU/mg and is a type II inhibitor. In addition, MAb 2A9 inhibits fVIII binding to VWF in an ELISA assay with a 50% inhibitory concentration (IC50) of 1 µg/ml. This is in comparison to the potent high-titer inhibitory anti-C2 MAb I89 (IC50 0.02 µg/ml) and a control non-inhibitory anti-A2 MAb ID4 (IC50 > 10 µg/ml).
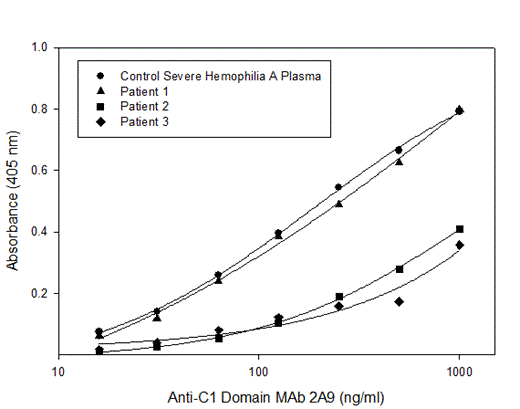
We tested 11 plasma samples from patients with congenital hemophilia with inhibitors in the Emory IRB-approved inhibitor bank. The plasma samples have inhibitory titers ranging from 1 - 188 BU/ml with a median inhibitory titer of 54 BU/ml and mean inhibitory titer 59 BU/ml. The plasmas were tested for the presence of antibodies that compete with anti-C1 domain MAb 2A9 using competition ELISA with fVIII as the antigen. Biotinylated MAb 2A9 was serially diluted and the concentration of antibody required to produce an absorbance at 405 nm of 0.3 was compared between control severe hemophilia A plasma and inhibitor plasma samples. Inhibitor plasma samples that reduced the ELISA titer of MAb 2A9 were considered a competitive inhibitor. Of the 11 inhibitor plasma samples, 4 were found to compete with MAb 2A9.
Our study demonstrates that anti-C1 domain antibodies are present in the plasma of patients with hemophilia A and inhibitors. Given the increasing evidence that the C1 domain is important in fVIII function it is likely that these anti-C1 antibodies are clinically relevant. Therefore, domains other than A2 and C2 need to be included in future studies of fVIII B cell epitopes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.