Abstract
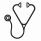
Background: CML is rare in pediatrics; <20 patients (pts) younger than 18 yrs are diagnosed in Germany annually. So far only imatinib (IMA) is fully licensed for treatment of pediatric CML while off-label use of 2nd generation tyrosine kinase inhibitors (TKIs) dasatinib (DASA) or nilotinib (NILO) is possible and is reimbursed by most German insurance companies by decision on an individual basis. Clinical features of CML at diagnosis differ age-dependently in minors in comparison to older adults [Pemmaraju N et al, Haematologica 2012, 97:1029; Kalmanti L et al, Ann Hematol 2014, 93:71] and also prominent differences could be demonstrated in controlled pediatric trials (CML-PAED I/II; French trial IV) when analyzing stage of disease (more advanced in pediatrics), spleen size (larger in pediatrics), and leukocyte number (higher in pediatrics) [Suttorp M et al, Hematology ASH Educ Program 2010:368; Millot F et al, J Clin Oncol 2011, 29:2817]. Also in children the up-front treatment of CML with IMA resulted in postponing stem cell transplantation (SCT). We here report on the course of CML in a greater cohort of pediatric and adolescent pts treated up-front by IMA as well as decisions taken at TKI failure due to intolerance or resistance.
Methods: At diagnosis pts were enrolled prospectively into the ongoing trial CML-PAED-II and treated with a standardized IMA dose (300/400/500 mg/sqm in chronic phase (CP); in accelerated phase (AP); in blast crisis (BC); respectively) under regular monitoring at three months intervals (Suttorp M et al, Bone Marrow Transplant 2008). Response rates (hematological, cytogenetic, molecular) and side effects were monitored following ELN guidelines. At treatment failure pts were switched to either a 2nd generation TKI or underwent HLA-matched (10/10 alleles) related or unrelated SCT on an individual basis [de la Fuente J et al, Br J Haematol 2014 Jun 30 epub ahead of print].
Results: As of April 2014 129 pts (57 fem, 72 male; mean age at diagnosis: 11.2 yrs; range: 1–17 yrs) could be analyzed. Stage of disease was CML-CP (n=120), CML-AP (n=5), and CML-BC (n=4; 2 myeloid / 2 lymphoid). With a median follow-up of 40 months (range: 1-96 mo) 77 pts (60%) of 129 pts still are on IMA while 48 pts experienced failure. In this situation only 18/48 opted for transplantation, while 30 pts were switched to a 2nd generation TKI (see Figure). 27/30 pts received DASA and 3/30 pts NILO as 2nd line TKI treatment. In this cohort, 16 pts also failed 2nd line TKI treatment (13 pts while on DASA and 3 pts while on NILO). After DASA failure, 8 pts went on to allogeneic SCT and 5 were treated with NILO as 3rd line treatment whereas after NILO failure 1 pt received DASA and 2 pts were transplanted. Out of 28 pts undergoing SCT 5 pts have died. Death was caused by transplant related complications (bleeding, infection, GvHD) in 3 pts (2 pts after failing 1st line TKI / 1 pt after 2nd line failure) and relapse of CML in 2 pts (diagnosed in CML-BC and transplanted after failing 1st line TKI). Out of 81 pts on ongoing IMA as 1st line treatment, 4 pts achieved PCR negativity for a minimum period of 2 years and have stopped the TKI maintaining complete molecular remission for so far 3, 5, 20, 46 mo, respectively [Moser O et al, Ped Blood Cancer 2014, May 9 epub ahead of print].
Conclusion: Side effects of IMA are well tolerated by the majority of pediatric pts. At failure of IMA only one third of the pts opted for SCT while the majority of treating physicians selected 2nd generation TKIs. For 2nd line treatment DASA was preferred to NILO. However, the precise role of these 2nd generation TKIs which presently are tested in activated / ongoing pediatric phase I/II trials still has to be clarified in minors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract