Abstract
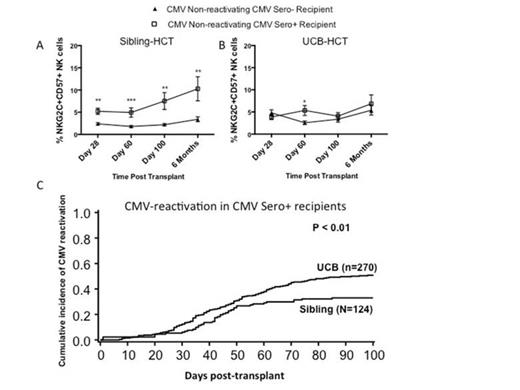
Natural killer (NK) cells can control malignant as well as virus-infected cells. We and others have recently described clonal expansion of NK cells expressing the activating receptor NKG2C, the maturation marker CD57, and self-educating inhibitory killer immunoglobulin-like receptors (KIRs) during the course of CMV infection. These NK cells persist long after the resolution of infection and are potent producers of interferon-γ, as such displaying adaptive or memory properties. While it is known that CMV drives a unique program of NK cell maturation, it is unclear how latent CMV may affect maturation of NK cells and other lymphocyte subsets following hematopoietic cell transplantation (HCT). 292 sibling and umbilical cord blood (UCB) HCT recipients were assessed for expression of NKG2C and CD57 on NK (total CD56+CD3- cells) or T cells (CD56+CD3+ and CD56-CD3+) during immune reconstitution. Data was analyzed based on pre-transplant CMV serostatus and post-HCT CMV-reactivation detected by weekly PCR screening (140 CMV seronegative [Sero-]; 83 CMV seropositive [Sero+] without reactivation; 69 Sero+ with reactivation). Phenotypic analyses were performed on blood at 28, 100, 180 and 365 days post-HCT. CMV Sero- recipients had low level NK cells co-expressing NKG2C and CD57 6 months post-transplant [3.8 ± 0.42%], while this population expanded in both CMV Sero+ recipients without reactivation [9.8 ± 2.1%; p=0.0044] and CMV Sero+ recipients with reactivation [12.6 ± 1.6%; p=0.0001]. Sero- recipients had low levels of CD56+CD3+ (2.7 ± 0.59%) and CD56-CD3+ T cells (0.11 ± 0.04%) co-expressing NKG2C and CD57 at 6 months. At 6 months CD56+CD3+ and CD56-CD3+ T cells co-expressing NKG2C and CD57 slightly increased in Sero+ recipients without reactivation [CD56+CD3+: 5.6% ± 1.6%; p=0.09 and CD56-CD3+: 0.3% ± 0.12%; p=0.2]. In contrast, NKG2C+CD57+ cells significantly expanded in Sero+ recipients with reactivation [CD56+CD3+: 11.7% ± 2.0%; p=0.0001 and CD56-CD3+: 0.6% ± 0.14%; p=0.002]. These T-cells expressed self-KIR (data not shown). We then studied the non-reactivating group to test whether latent CMV exposed NK and T cells in adult donors differed from UCB HCT where the graft is CMV naïve. After sibling HCT at 100 days, Sero+ recipients (n=43) showed significant increases of NK cells co-expressing NKG2C and CD57 (p<0.01; Figure 1A) and CD56+CD3+ T cell populations (p=0.04, data not shown) compared to Sero- recipients (n=78). In marked contrast, no significant differences in NK or T cells co-expressing NKG2C and CD57 were observed post-HCT in Sero+ UCB recipients (n=40) compared to Sero- recipients (n=62) (Figure 1B). Therefore, adult donor adaptive NK cells can be transferred from the donor and expand in the recipient while these same cells require CMV reactivation post HCT to develop from UCB grafts. We then studied 674 sibling and UCB HCT for CMV reactivation and NK adaptive responses. Among Sero+ recipients (n=394), increased NK cells co-expressing NKG2C and CD57 following sibling but not UCB HCT (Figure 1AB) was accompanied by a decreased incidence of CMV reactivation [Sibling: n=124 (33% CMV reactivation); UCB: n=270 (51%)] (p<0.01) (Figure 1C). Multivariate analysis confirmed an independent increased relative risk (RR) of CMV reactivation in UCB vs. sibling recipients (RR=1.6 [1.1-2.3], p<0.01). Taken together, our data demonstrate that Sero+ recipients of adult donor grafts, even without CMV reactivation, can expand NKG2C+CD57+ NK cells. This indicates that such adaptive or memory cells are transplantable, contrasting the UCB transplant setting in which clinical CMV reactivation is required to induce adaptive NK and T cell immunity. The physiologic importance of these findings is highlighted by differences in CMV reactivation in sibling vs. UCB transplant for Sero+ recipients who are at risk of CMV reactivation. Our findings in human patients parallel experiments of Ly49H+ NK cell transfer protecting mice against murine CMV. Clinically, strategies to expand adaptive NK and T cells without CMV infection may enhance immune reconstitution after HCT and decrease the morbidity associated with CMV reactivation.
Miller:Coronado: Speakers Bureau; BioSciences: Membership on an entity's Board of Directors or advisory committees; SAB: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract