Abstract
Background: JAK2 inhibitors have been shown to improve symptoms and produce durable reductions in splenomegaly in patients with myelofibrosis (MF), and ruxolitinib has been shown to improve survival in MF patients (Cervantes et al., 2013; Verstovsek et al., 2013). Current prognostic models such as DIPSS plus (Gangat et al., 2011) predict survival in MF based on clinical, laboratory, and cytogenetic information, but their value in predicting clinical response or survival during treatment with JAK2 inhibitors remains unknown. We hypothesized that clinical features such as bone marrow fibrosis and splenomegaly may have independent effects on therapy response. Therefore, we conducted a retrospective analysis to create a new model to risk stratify patients with respect to their likelihood of responding to oral JAK2-inhibitor therapy.
Methods: We studied a cohort of 203 patients with bone marrow biopsy-proven MF seen at University of Michigan, Stanford University, and Mayo Clinic in Scottsdale, AZ. These patients were all treated with ruxolitinib or an experimental JAK2 inhibitor. Our primary endpoint was defined as IWG-MRT criteria for splenic response by palpation (Tefferi et al., 2013). Response in patients with spleen size of less than 5 cm was defined as complete resolution of splenomegaly. Of the 203 patients studied, splenic response was evaluated after 3 months of therapy in 167 patients and after 6 months of therapy in 138 patients; 127 patients were in both groups. A logistic regression was performed to identify factors that would predict clinical response.
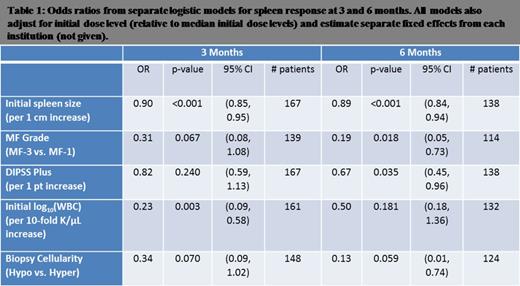
Results: The following characteristics were significantly associated with spleen response at 3 and 6 months: initial spleen size, European consensus criteria grading of MF on bone marrow biopsy, initial DIPSS plus score, and initial WBC count. Cellularity on marrow biopsy was not significant.
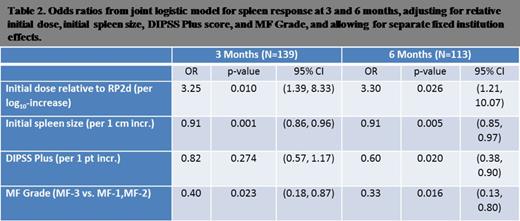
We enriched a baseline logistic model of initial dose of oral JAK2 therapy and DIPSS plus score with additional prognostic factors. We found the following clinical characteristics to be jointly associated with splenic response: normalized initial dose of oral JAK2 inhibitor, initial spleen size, DIPSS plus score, degree of fibrosis by European consensus criteria. Duration of disease from time of diagnosis to time of treatment initiation was not prognostic for splenic response.
We used this model to calculate the probability of splenic response based on a risk score:
Risk score = –1.18(Dose) + 0.09(Initial Spleen Size in cm) + 0.20(DIPSS-plus Points) +0.92 (if fibrosis is MF-3).
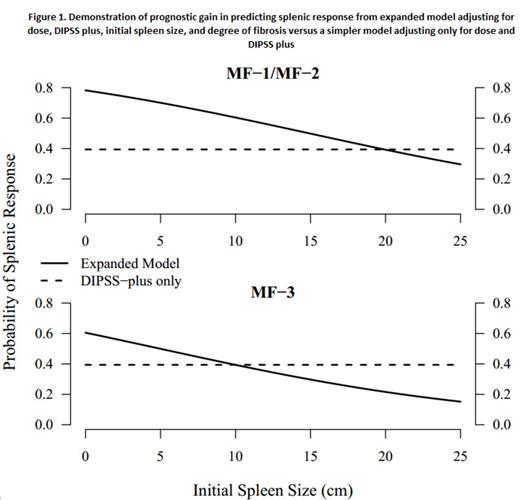
The probability at 6 months can then be calculated from the risk score as follows: [1/(1+e(risk score-2.6))]. The figure demonstrates the prognostic gain of our model over a model based on DIPSS plus alone.
Conclusion: With this observational study, we propose a predictive model which may serve as a clinical tool to identify which patients are most likely to benefit from JAK2 inhibitor-based therapies. Further validation in independent data sets will be required before this model can be more widely applied.
Mesa:Incyte Corporation, CTI, NPS Pharma, Inc., Gilead Science Inc., Celgene: Research Funding. Gotlib:Incyte: Consultancy, Honoraria, Research Funding, Travel Reimbursement Other; Gilead: Research Funding; Sanofi: Research Funding; Novartis: Research Funding, Travel Reimbursement, Travel Reimbursement Other. Talpaz:ARIAD Pharmaceuticals, Inc., BMS, Sanofi, Incyte, Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.