Abstract
BACKGROUND: Ruxolitinib (RUX) is a potent JAK1/JAK2 inhibitor that has demonstrated superiority over traditional therapies in the treatment of MF. In the 2 phase 3 COMFORT studies, RUX demonstrated rapid and durable reductions in splenomegaly, improved MF-related symptoms and quality of life measures, and improved survival compared with placebo and best available therapy. The aim of EXPAND was to evaluate safety of RUX and establish a maximum safe starting dose (MSSD) in thrombocytopenic MF patients (pts).
METHODS: EXPAND is a phase 1b, dose-finding study (NCT01317875) in MF pts with baseline platelet counts (PLTs) 50 to 99 × 109/L. Pts with ≥ 1 episode of PLTs < 100 × 109/L during screening were eligible. A Bayesian logistic regression model was used to guide dose-escalation decisions; intra-pt dose modification was allowed. The study consists of 2 phases: dose escalation and safety expansion. In the dose-escalation phase, doses of RUX investigated included 5 mg bid, 5 mg AM/10 mg PM, 10 mg bid, 10 mg AM/15 mg PM, and 15 mg bid (cohorts C1-5). Pts were assigned to 2 strata based on their baseline PLTs: S1: 75 to 99 × 109/L; S2: 50 to 74 × 109/L. Dose levels in S2 were open only if that dose and the next were deemed safe in S1. In the safety-expansion phase, 20 additional pts (10 per stratum) will be treated at the stratum MSSD.
RESULTS: To date, 34 pts (S1, n = 21; S2, n = 13) received treatment across 8 cohorts (S1: C1, n = 4; C2, n = 3; C3, n = 4; C4, n = 4; C5, n = 6; S2: C1, n = 3; C2, n = 3; C3, n = 7). Baseline characteristics were generally balanced across strata: 68% of pts were ≥ 65 y, 41% male, 74% had PMF (24% PPV-MF, 3% PET-MF), and 62% had high-risk MF; spleen length ranged from 5 to 33 cm below the costal margin. Baseline hemoglobin ranged from 83 to 138 g/L in S1 and 77 to 133 g/L in S2; PLTs ranged from 56 to 112 × 109/L in S1 and 47 to 100 × 109/L in S2. As of data cutoff, 9 (43%) and 7 (54%) pts were still receiving treatment in S1 and S2, respectively. The primary reasons for discontinuation were adverse events (AEs; S1, 19% [4]; S2, 23% [3]), deaths (9.5% [2]; 7.7% [1]), disease progression (4.8% [1]; 0), and pt decision (0; 7.7% [1]).
Reported AEs were consistent with the known safety profile of RUX (Table). 94% experienced grade 3/4 AEs. AEs leading to discontinuation were all grade 3/4 (S1, 24%; S2, 31%); besides thrombocytopenia (S2, n = 2; S1, n = 0), no other AE led to treatment discontinuation in > 1 pt in either stratum. Three pts in S1 and 1 in S2 had grade 3/4 hemorrhage; 24 pts had AEs requiring dose reduction, most commonly anemia (S1, 14%; S2, 8%) and thrombocytopenia (38%; 77%); the remainder occurred in 1 pt each. Hemoglobin decreases were generally balanced across dose levels: no pt had grade 4 (all grade, 68%; grade 3, 50%). PLT decreases were more frequent with increasing dose: 2 pts (S1/C2) and 3 pts (1 in S2/C2 and 2 in S2/C3) had grade 4 (all grade, 94%; grade 3/4, 53%). Reasons for death included cardiac failure (S1), sudden death (S1), and multi-organ failure (S2).
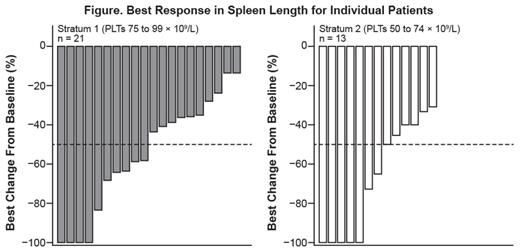
Of 34 pts, 33 (97%) had reductions in palpable spleen length, with complete resolution in 9 pts; 17 pts (50%) had a ≥ 50% reduction as their best response on study (Figure). Results for changes in symptom scores will be presented.
CONCLUSIONS: Starting doses of up to 15 mg bid have been tolerated well in MF pts with low PLTs, and the safety in the escalation phase is consistent with findings from previous studies. While the study is still ongoing and proceeding into the expansion phase, preliminary evaluations indicate 15 and 10 mg bid as MSSDs for pts with PLT 75 to 99 × 109/L and 50 to 74 × 109/L, respectively.
AEs in ≥ 10% of Pts in Either Stratum
| % . | S1 n = 21 . | S2 n = 13 . | ||
|---|---|---|---|---|
| All Grade | Grade 3/4 | All Grade | Grade 3/4 | |
| Abdominal pain | 10 | 5 | 8 | 0 |
| Abdominal pain upper | 24 | 0 | 0 | 0 |
| Anemia | 57 | 43 | 31 | 23 |
| Arthralgia | 19 | 0 | 8 | 0 |
| Asthenia | 19 | 10 | 23 | 0 |
| Back pain | 14 | 5 | 23 | 8 |
| Bronchitis | 0 | 0 | 23 | 0 |
| Contusion | 5 | 0 | 23 | 0 |
| Cough | 10 | 0 | 38 | 0 |
| Decreased appetite | 14 | 0 | 8 | 0 |
| Diarrhea | 33 | 0 | 23 | 0 |
| Dizziness | 19 | 0 | 8 | 0 |
| Dyspnea | 5 | 0 | 15 | 0 |
| Epistaxis | 24 | 5 | 0 | 0 |
| Fatigue | 14 | 5 | 8 | 0 |
| Headache | 19 | 0 | 23 | 0 |
| Hyperuricemia | 0 | 0 | 15 | 8 |
| Influenza | 0 | 0 | 15 | 0 |
| Influenza-like illness | 10 | 0 | 0 | 0 |
| Insomnia | 14 | 0 | 8 | 0 |
| Muscle spasm | 14 | 0 | 8 | 0 |
| Musculoskeletal chest pain | 10 | 0 | 0 | 0 |
| Nasopharyngitis | 10 | 0 | 23 | 0 |
| Nausea | 19 | 0 | 8 | 0 |
| Edema peripheral | 14 | 5 | 15 | 0 |
| Oropharyngeal pain | 14 | 0 | 15 | 0 |
| Pain in extremity | 5 | 0 | 23 | 0 |
| Pollakiuria | 10 | 0 | 0 | 0 |
| Procedural pain | 10 | 0 | 0 | 0 |
| Pruritus | 0 | 0 | 23 | 0 |
| Pyrexia | 10 | 0 | 23 | 0 |
| Skin lesion | 10 | 0 | 0 | 0 |
| Splenomegaly | 10 | 5 | 0 | 0 |
| Thrombocytopeniaa | 81 | 67 | 85 | 85 |
| Urinary tract infection | 10 | 0 | 8 | 0 |
| Vomiting | 14 | 0 | 8 | 0 |
| Weight increased | 10 | 0 | 0 | 0 |
| % . | S1 n = 21 . | S2 n = 13 . | ||
|---|---|---|---|---|
| All Grade | Grade 3/4 | All Grade | Grade 3/4 | |
| Abdominal pain | 10 | 5 | 8 | 0 |
| Abdominal pain upper | 24 | 0 | 0 | 0 |
| Anemia | 57 | 43 | 31 | 23 |
| Arthralgia | 19 | 0 | 8 | 0 |
| Asthenia | 19 | 10 | 23 | 0 |
| Back pain | 14 | 5 | 23 | 8 |
| Bronchitis | 0 | 0 | 23 | 0 |
| Contusion | 5 | 0 | 23 | 0 |
| Cough | 10 | 0 | 38 | 0 |
| Decreased appetite | 14 | 0 | 8 | 0 |
| Diarrhea | 33 | 0 | 23 | 0 |
| Dizziness | 19 | 0 | 8 | 0 |
| Dyspnea | 5 | 0 | 15 | 0 |
| Epistaxis | 24 | 5 | 0 | 0 |
| Fatigue | 14 | 5 | 8 | 0 |
| Headache | 19 | 0 | 23 | 0 |
| Hyperuricemia | 0 | 0 | 15 | 8 |
| Influenza | 0 | 0 | 15 | 0 |
| Influenza-like illness | 10 | 0 | 0 | 0 |
| Insomnia | 14 | 0 | 8 | 0 |
| Muscle spasm | 14 | 0 | 8 | 0 |
| Musculoskeletal chest pain | 10 | 0 | 0 | 0 |
| Nasopharyngitis | 10 | 0 | 23 | 0 |
| Nausea | 19 | 0 | 8 | 0 |
| Edema peripheral | 14 | 5 | 15 | 0 |
| Oropharyngeal pain | 14 | 0 | 15 | 0 |
| Pain in extremity | 5 | 0 | 23 | 0 |
| Pollakiuria | 10 | 0 | 0 | 0 |
| Procedural pain | 10 | 0 | 0 | 0 |
| Pruritus | 0 | 0 | 23 | 0 |
| Pyrexia | 10 | 0 | 23 | 0 |
| Skin lesion | 10 | 0 | 0 | 0 |
| Splenomegaly | 10 | 5 | 0 | 0 |
| Thrombocytopeniaa | 81 | 67 | 85 | 85 |
| Urinary tract infection | 10 | 0 | 8 | 0 |
| Vomiting | 14 | 0 | 8 | 0 |
| Weight increased | 10 | 0 | 0 | 0 |
a All pts had grade1/2 thrombocytopenia at study entry.
te Boekhorst:Novartis: Consultancy. Harrison:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria; CTI: Consultancy, Honoraria; Gilead: Honoraria; SBio: Consultancy. Gisslinger:AOP ORPHA: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen Cilag: Honoraria, Speakers Bureau; Geron: Consultancy; Sanofi Aventis: Consultancy. Niederwieser:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gentium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Stalbovskaya:Novartis: Employment, Equity Ownership. Atienza:Novartis: Employment. Gopalakrishna:Novartis: Employment. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal