Abstract
CLL with deletion 17p (17p-) or refractory to fludarabine (F)-based regimens is characterized by poor prognosis. The cooperative French/German CLL2O study aimed at achieving deep and durable response in this population by combining alemtuzumab (A) and dexamethasone (D) induction, followed by consolidation with A maintenance or allogeneic stem-cell transplantation (allo-SCT).
Induction treatment consisted of subcutaneous A (30mg, 3x weekly) combined with oral D (40 mg days 1-4 and 15-18), both at 28 day cycles, and prophylactic pegfilgrastim 6 mg on days 1 and 15. If at least SD was achieved after 3 cycles, consolidation was scheduled with either allo-SCT or A maintenance (30mg every 2 weeks for up to 2 years), at discretion of pt and physician.
Between January 2008 and December 2011, 131 eligible pts were enrolled at 26 centers. Pts were generally subdivided for this analysis into three cohorts: 17p- 1st line, 17p- relapsed (not refractory) and refractory (i.e. no response or relapse within 6 months) to F-based or similar (i.e. pentostatin, cladribine, bendamustine) therapy. All three cohorts where characterized by high-risk baseline disease features (detailed in Table).
During induction, a total of 467 non-hematologic AEs were recorded, predominantly (79%) of minor grade, while 36%, 43%, and 50% of pts in the 3 cohorts had at least one event of grade 3 or higher (Table).
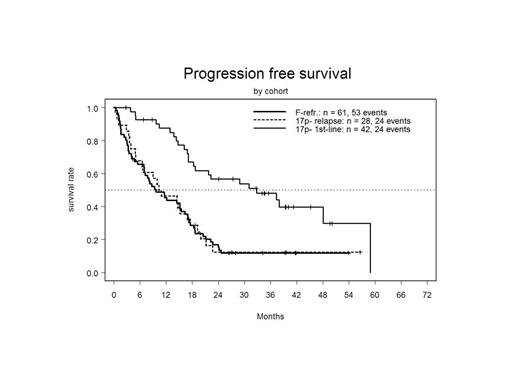
ORR (best response) was high in all three cohorts, but CRs were rarely observed outside the 17p- 1st line cohort (Table). Correspondingly, there were marked differences in PFS and OS between the three cohorts with far better outcome in the 17p- 1st line group (see Table and Figure 1).
Consolidation treatment was performed as A maintenance (median duration 42 weeks, range 2 – 112.4) in 37%, and allo-SCT in 25%, with a median age of 69 and 57 y in these subgroups. The main reasons for going off-study without consolidation were death due to infection in 19 patients (15%), largely from the F-refractory cohort (n=16), with the majority being non-responders to study treatment (n=11); CLL progression (10%), and other toxicity (9%). Five pts who did not receive immediate consolidation treatment per protocol later underwent allo-SCT, 2 of these have died (sepsis, GvHD).
During A maintenance, grade 3/4 toxicity consisted of neutropenia in 47% and thrombocytopenia in 12%. Serious (grade 3/4) non-CMV infection occurred in 17%, 10%, 13% in the 3 cohorts.
When comparing PFS between A maintenance and allo-SCT, there were 41 (84%) and 16 (48%) events, respectively, significantly favoring SCT (Figure 2). Six pts who started A maintenance later underwent allo-SCT, 4 of them after relapse. After median follow up of 20 months from allo-SCT, 12 pts have died, 7 because of non-relapse mortality and 5 subsequent to CLL progression.
In conclusion, the combination of A and D, followed by A maintenance or allo-SCT showed high response rates in ultra high-risk CLL with expected toxicity. For 17p- 1st line treatment, the results compare favorably to FCR (CLL8: ORR 68%, median PFS 11.3 mo). On the other hand, in F-refractory and 17p- relapsed CLL, the high ORR did not translate into prolongation of PFS. Allo-SCT appears to offer superior disease control in eligible patients despite prior A exposure. Overall, this mature trial may serve as a historical benchmark for comparison of novel agents in ultra high-risk CLL.
| Parameter . | 17p- 1st line . | 17p- relapsed . | F-refractory . |
|---|---|---|---|
| Number of patients | 42 | 28 | 61 |
| Median age (yrs) | 66.5 | 64 | 66 |
| Binet C (%) | 45 | 57 | 77 |
| B symptoms (%) | 40 | 32 | 31 |
| ECOG 1/2 (%) | 38 | 39 | 56 |
| Median thymidine kinase (U/l) | 35 | 48.1 | 27.6 |
| Median β2MG (mg/dl) | 3.8 | 5.1 | 4.7 |
| Unmutated IGHV (%) | 90 | 93 | 87 |
| 17p- (%) | 100 | 100 | 49 |
| Prior lines (median) | n.a. | 2 | 3 |
| Prior rituximab (%) | n.a. | 71 | 93 |
| Parameter . | 17p- 1st line . | 17p- relapsed . | F-refractory . |
|---|---|---|---|
| Number of patients | 42 | 28 | 61 |
| Median age (yrs) | 66.5 | 64 | 66 |
| Binet C (%) | 45 | 57 | 77 |
| B symptoms (%) | 40 | 32 | 31 |
| ECOG 1/2 (%) | 38 | 39 | 56 |
| Median thymidine kinase (U/l) | 35 | 48.1 | 27.6 |
| Median β2MG (mg/dl) | 3.8 | 5.1 | 4.7 |
| Unmutated IGHV (%) | 90 | 93 | 87 |
| 17p- (%) | 100 | 100 | 49 |
| Prior lines (median) | n.a. | 2 | 3 |
| Prior rituximab (%) | n.a. | 71 | 93 |
AEs during induction (grade 3/4)
| All AEs (%) | 36 | 43 | 50 |
| Neutropenia (%) | 24 | 36 | 67 |
| Anemia (%) | 14 | 36 | 21 |
| Thrombocytopenia (%) | 12 | 39 | 31 |
| Non-CMV infection (%) | 19 | 36 | 36 |
| CMV infection (%) | 7 | 0 | 2 |
| All AEs (%) | 36 | 43 | 50 |
| Neutropenia (%) | 24 | 36 | 67 |
| Anemia (%) | 14 | 36 | 21 |
| Thrombocytopenia (%) | 12 | 39 | 31 |
| Non-CMV infection (%) | 19 | 36 | 36 |
| CMV infection (%) | 7 | 0 | 2 |
Efficacy (median follow-up 41.3 mo)
| ORR (%) . | 97 . | 79 . | 69 . |
|---|---|---|---|
| CR (%) | 21 | 4 | 3 |
| Median PFS (mo) | 32.8 | 10.3 | 9.7 |
| Median OS (mo) | >60.0 | 21.4 | 17.3 |
| ORR (%) . | 97 . | 79 . | 69 . |
|---|---|---|---|
| CR (%) | 21 | 4 | 3 |
| Median PFS (mo) | 32.8 | 10.3 | 9.7 |
| Median OS (mo) | >60.0 | 21.4 | 17.3 |
Stilgenbauer:Amgen: Honoraria, Research Funding; Genzyme: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.