Abstract
Background: Treatment with oral melphalan and dexamethasone has been adopted as the standard of care for newly diagnosed patients with immunoglobulin light chain (AL) amyloidosis not eligible for high-dose melphalan and stem cell transplantation (HDM/SCT). However, new treatment options are still needed for this patient population.
Aim: Based on the activity of IMiDs® immunomodulatory drugs in relapsed/refractory AL amyloidosis, we designed a multicenter prospective, phase II trial with lenalidomide, dexamethasone and cyclophosphamide for newly diagnosed patients not eligible for autologous transplant.
Patients and methods: The main inclusion criteria were newly diagnosed AL amyloidosis confirmed by biopsy, significant organ involvement, cardiac ejection fraction over 50%, serum creatinine below 3 mg/dL, and not being eligible for autologous transplant. Treatment schedule consisted of 6 cycles of lenalidomide at 15 mg orally (po) on days 1 to 21, dexamethasone at 20 mg po on days 1 to 4 and 9 to 12, and cyclophosphamide at 300 mg/m2 intravenously (iv) on days 1 and 8 every 28 days, followed by 6 more cycles of lenalidomide at the same dose, dexamethasone at 20 mg po on days 1 to 4 and cyclophosphamide 300 mg/m2 iv on day 1. After these 12 cycles, maintenance with lenalidomide (10 mg po on days 1 to 21) and dexamethasone (20 mg po on days 1 to 4) was planned for three additional years or until discontinuation due to intolerance or disease progression. All patients received prophylaxis of thromboembolic events with oral aspirin (100 mg po daily) or subcutaneous low-molecular-weight heparin. The primary endpoint was hematologic response. Diagnosis of AL amyloidosis, definition of organ involvement and response criteria followed the Consensus Opinion from the Xth International Symposium on Amyloid and Amyloidosis (Gertz et al, Am J Hematol 2005), adding the very good partial response category included in the recently reported criteria (Palladini et al, J Clin Oncol 2012). Assessments of response were performed at the beginning of each cycle during the treatment period and every 3 months during the maintenance phase.
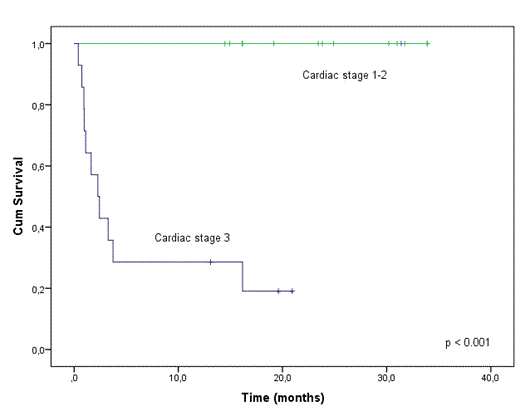
Results: From September 2010 to December 2012, 28 patients were enrolled in the study. Twenty-three patients had cardiac involvement, with cardiac stage 3 in 14 of them. The overall hematologic response rate was 46%, including 7 patients (25%) with complete response, 5 patients (18%) with very good partial response and 1 patient (3%) with partial response. The organ response rate was 46% and was only observed among patients who achieved hematologic response. The organ response was reached in 10 (43%) of the 23 patients with renal involvement and 6 (26%) of the 23 patients with cardiac involvement. In 4 patients lenalidomide was reduced or discontinued due to toxicity. A therapy-related serious adverse event was reported in 6 patients. No significant cytopenias and no second primary malignancies (SPM) were observed. So far, 11 patients remain on the study. Seventeen (60%) have been discontinued mostly due to death secondary to cardiac or renal AL-related late events (8 patients), progression or lack of response (4), and toxicity (3). After a median follow up of 24 months, the median PFS and OS have not been reached, being significantly longer in responders. The estimated probability of PFS and OS for responders was 92% at 34 months. In contrast, the median PFS and OS for non-responders were only 1.9 and 2.4 months, respectively. Finally, according to Mayo Clinic risk stratification based on cardiac biomarkers, median OS for patients with stage I-II has not been reached (the 2-year estimate was 100%), and was 2.2 months for stage III (p <0.001; Figure 1 ).
Conclusions: Our results support the efficacy and safety of the combination lenalidomide, dexamethasone and cyclophosphamide as a new treatment option for patients with AL amyloidosis not eligible for ASCT, without the risk of neuropathy associated with bortezomib-based therapies. However, this regimen should be preferably used when the organ function is still preserved since patients with advanced stage disease, particularly those with severe cardiac involvement, are unlikely to benefit.
Blade:Janssen, Celgene and Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Off Label Use: Lenalidomide. First-line therapy in combination for AL amyloidosis.. Oriol:Janssen and Celgene: Honoraria. Lahuerta:Celgene: Honoraria; Janssen: Honoraria. Mateos:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. de la Rubia:Janssen and Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Fernandez de Larrea:Janssen and Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. San Miguel:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees; Millenium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cibeira:Janssen and Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.