Abstract
Background: It is now well established that the immune modulator (IMiD) drugs thalidomide (Thal), lenalidomide (Len) and pomalidomide (Pom) bind a specific pocket in the protein, cereblon (CRBN) that is required for MM cytotoxicity after IMiD treatment. Recently, we reported 46 CRBN binding proteins which were altered by Len, two of which were the transcription factors Ikaros (IKZF1) and Aiolis (IKZF3), important regulators of B and T cell development. Others had previously reported the same finding and went further by demonstrating that following binding of IMiD drugs, CRBN accumulates and accentuates the ubiquitination and subsequent proteasomal degradation of these two binding proteins. The resulting degradation and depletion of IKZF proteins has the simultaneous effect of downregulating IRF4 and MYC leading to MM cell death while simultaneously enhancing transcription of interleukin 2 and activating the immune compartment.
Methods: To further explore this phenomenon and its relationship to drug sensitivity we first created two stable MM cell lines (H929IKZF1Luc, 8226IKZF1Luc) expressing an IKZF1-luciferase (luc) fusion gene and tested for sensitivity to Len alone and in combination with dexamethasone (Dex), bortezomib (Bor) or the histone deacetylase inhibitor SAHA. Next, we constructed an adenoviral vector expressing IKZF1-luc and used this to transfect 16 MM cell lines, confirmed expression and tested sensitivity to Len by MTT (Figure 1).
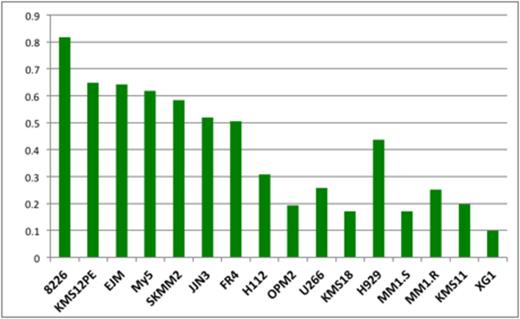
Results: We first examined the stable cell lines and measured luc expression after exposure to all three IMiD drugs. With all IMiD drugs luc dramatically decreased in sensitive H929 cells. IKZF1 depletion was both time and dose dependent. Using this surrogate marker cell line assay, luc is most efficiently degraded by Pom which is twice as potent as Len, but a thousand times more potent than Thal (Concentration of drug required to reduce luc expression by 50%, Pom: 4.9nM, Len: 10.2 nM, and Thal: 4,795 nM). We then tested all 16 cell lines with Len 2uM for 5 hours and show a strong correlation of IKZF-luc depletion with sensitivity.
Luc levels 5 hours after Len treatment at 2uM. We divided the cell lines into three groups: 1) Len resistant: 8226, KMS12PE, My5, SKMM2, JJN3, FR4, H112; 2) Len intermediate response: EJM, OPM2, U266, KMS18; 3) Len sensitive: H929, MM1.S, MM1.R, KMS11, XG1;
Luc levels 5 hours after Len treatment at 2uM. We divided the cell lines into three groups: 1) Len resistant: 8226, KMS12PE, My5, SKMM2, JJN3, FR4, H112; 2) Len intermediate response: EJM, OPM2, U266, KMS18; 3) Len sensitive: H929, MM1.S, MM1.R, KMS11, XG1;
We next performed western blots on 9 MM cell lines and demonstrate that IKZF1 levels were lowest in 3 of 5 resistant MM cell lines and normal in all 4 sensitive lines studied. The cell line (FR4) with the lowest levels of IKZF1 harbors a damaging mutation of IZKF1 as well as a translocation which upregulates IRF4, an IKZF target. Of the remaining two resistant lines one has low levels of Cereblon. In one line the mechanism of IMiD resistance could not be established. Finally, we were curious about the dichotomy of the IMiD biology which seemingly has an absolute requirement for a functional proteasome for successful IMiD cytotoxicity which is at variance with clinical reports of synergy between the IMiDs and the proteasome inhibitors (PIs) Bor and carfilzomib (Car). To explore this, we treated the 8226IKZF1Luc cell line with Len alone or in combination with increasing concentrations of Bor (6 nM) or Car (40nM) and found that both totally blocked IKZF1 fusion protein degradation promoted by Len, beginning as soon as 1 hour at 100nM Bor or after 3 hours at 40nM treatment. Conversely, in low concentration of Bor (<4nM) no IKZF1 degradation blocking was observed in the Luc assay and Bor remained lethal to the MM cells. In that light with lower levels of Bor (<3nM) even varying the sequencing of MM cell exposure to the two drug classes did not block cytotoxicity. Other MM active drug Dex or SAHA, blocking the aggresome, had no significant inhibition for Len-induced IKZF1 fusion protein degradation.
Conclusion: These results validate the central role for the CRBN-IKZF-IRF4 axis proteins in IMiD response and show a strong correlation of IZKF1 expression and the ability to degrade IKZF1 with sensitivity. Our data provide interesting and perhaps disconcerting biologic data suggesting that the timing and dosing of clinical use of IMiDs and PI treatment in combination should be carefully evaluated to prevent antagonism of effect but suggest in vitro that a therapeutic window exists at which proteasome inhibitors can be active without blocking IMiD activity.
Stewart:Sanofi: Consultancy; Array BioPharma: Consultancy; Bristol Myers Squibb: Consultancy; Celgene: Consultancy; Novartis: Consultancy; Takeda Pharmaceuticals International Co.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.