Abstract
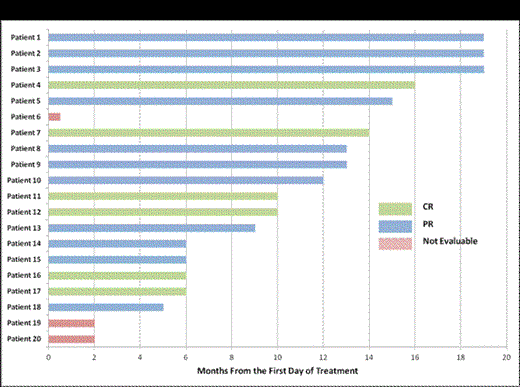
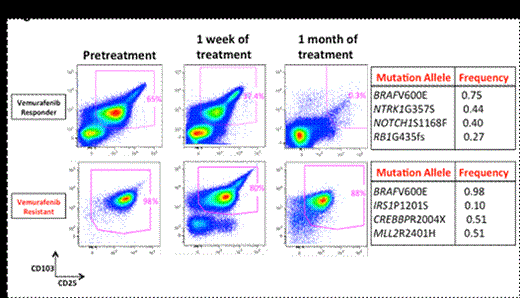
Background: Although treatment with purine analogs is associated with a high response rate, hairy cell leukemia (HCL) remains incurable with a 30-40% relapse rate. For these patients (pts) and those intolerant to purine analogs, novel therapies are needed. The major finding that BRAFV600E mutations occur in 98% of HCL suggests BRAF as a promising therapeutic target. We therefore designed a phase II trial to (1) determine the clinical efficacy of the BRAF inhibitor vemurafenib in pts with relapsed/ refractory HCL and (2) identify biologic determinants of response and resistance to vemurafenib in HCL. Patients and Methods: Pts with BRAF mutant HCL who were refractory or resistant to purine analogs, or who had ≥2 relapses with an indication for treatment (ANC ≤1.0, HGB ≤10, or PLT ≤100K) were enrolled. Eligible pts received vemurafenib 960mg twice daily for 3 months. Bone marrow (BM) evaluations were performed after 3 months to assess response. Pts with partial (PR) or complete response (CR) with detectable minimal residual disease (MRD) were allowed to receive vemurafenib for up to 3 additional months. The primary endpoint of the study was overall response rate (ORR: CR + PR). Using a Simon’s mini-max two-stage design, if at least 4 of the 19 pts (≥20%) achieve ORR in the first stage, an additional 17 patients will be accrued to the second stage. If 11 or more pts achieve ORR out of the 36 pts, the study would be considered worthy of further investigation. Serial peripheral blood and/or BM samples were collected during the study for quantitative BRAF mutant allele burden, serum cytokine, multiparameter flow cytometry, and targeted next-generation sequencing analysis of a 350-gene panel to detect potential predictors of resistance and identify genes collaborating with BRAF mutations in HCL. Results: 22 pts have been enrolled and 20 pts received treatment. The median age was 61 years (range 44-77), and the median number of prior treatments was 3.5 (range 1-7). 20 pts are evaluable for toxicity and 17 patients for disease response with a median follow up of 10 months (range 2-19). The most common adverse events were rash (40%, Gr1-2), arthralgia (30%, Gr1-2), photosensitivity (20%, Gr1), and pruritis (15%, Gr1). Three pts developed squamous cell carcinoma (SCC), all of whom had previous history of SCC. All patients were able to complete the intended treatment (3 cycles: 11 pts, >3 cycles: 6 pts). All 17 evaluable pts achieved complete hematologic recovery with an overall response rate (ORR) of 100%. 6 pts achieved CR (4 MRD- and 2 MRD+) and 11 pts achieved PR with very minimal disease (Figure 1). Responses were rapid with reduction of circulating hairy cells within 24 hours and normalization of sCD25 within 2-3 weeks (Figure 2). This correlated with dephosphorylation of ERK at 1 month. Several additional inflammatory cytokines correlated with the leukemic cell burden identifying novel tumor markers in HCL including sTNF-R2, sIL-1R2, and sIL4R.
Genetic analysis identified a median of 3 (range 0-5) somatic mutations co-existing with the BRAFV600E mutation in HCL including recurrent mutations in MLL2 and CREBBP (Figure 2). Several of these alterations were present as minor clones identifying a clonal architecture in HCL which was not previously appreciated.
One pt was found to have de novo resistance to vemurafenib. Genetic analysis identified that this pt’s pretreatment HCL cells harbored a previously undescribed missense mutation in IRS1 (IRS1P1201S) in addition to BRAFV600E mutation. Given prior knowledge that IRS1 (Insulin Receptor Substrate 1) activates both MAP kinase and PI3K-AKT signaling, we compared the effects of expression of wildtype and mutant IRS1 cDNAs on signaling. This revealed robust activation of PI3K-AKT signaling induced by IRS1P1201S-mutant cells relative to wildtype.
Conclusions: While longer follow-up is needed to assess the durability of the response, our results indicate that vemurafenib has potent antitumor activity in pts with relapsed/refractory BRAF mutant HCL. These data confirm the MAP kinase pathway as a rational promising therapeutic target in HCL and identify activation of signaling pathways parallel to the MAP kinase pathway as a first mechanism of RAF inhibitor resistance in a hematological malignancy. Subsequent studies will be required to prove that inhibition of B-raf may be the best initial therapy in HCL pts who require treatment.
Park:Genentech: Research Funding. Off Label Use: Vemurafenib in hairy cell leukemia. Rosen:Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.