Abstract
Rationale:
Allogeneic hematopoietic stem cell transplantation (HSCT) remains the most effective therapy for patients with acute myeloid leukemia (AML). The graft-versus-leukemia effect (GVL), mediated by the engrafted T lymphocytes targeting leukemic cells, is thought to play an important role in affecting the overall outcome of patients with AML. Umbilical cord blood (UCB) has emerged as an alternative and effective source of hematopoietic stem cells in high risk patients. We previously reported the hematopoietic reconstitution and clinical outcomes in 45 patients undergoing haplo-cord transplant, a novel approach which combines haploidentical and cord blood grafts to avoid delayed hematopoietic recovery after cord blood transplant (Liu H,et al. Blood. 2011;118(24):6438-45). However, very little is known about immune reconstitution of cord blood cells and whether the emerging immune repertoire correlates with clinical outcome. The great majority of T cell receptors in T lymphocytes are heterodimers of alpha (TCRA) and beta (TCRB) subunits. Somatic recombination combining the VJ (alpha) and VDJ (beta) segments results in an astronomical functional diversity and complexity of TCR receptors, and makes the characterization of their functions a tremendously complex process. To obtain insights into the T-cell repertoire, we have utilized next-generation sequencing (NGS) technology to comprehensively characterize T-cell kinetics and diversity following haplo-cord transplantation.
Methods:
We evaluated the emerging T-cell repertoire in 10 patients (pts) with high-risk AML enrolled on a clinical trial of haplo-cord transplantation at the University of Chicago. The median age of the pts was 57 years old (range: 26-67) and 3 pts had active disease at the time of transplant. The median UCB dose was 1.6x107 TNC/kg with HLA cord matching of 4/6 in 3 pts and 5/6 or 6/6 in 7 pts. The median overall and disease free survival were 2.4 and 2.2 years, respectively. cDNA was generated from mRNA isolated from peripheral blood mononuclear cells prior to and at sequential time points 30, 100, 180 and 365 days post haplo-cord transplant were sequenced the samples with Ion Personal Genome Machine (PGM) Sequencer and a 400-bp reading kit. We analyzed the sequences by applying a recently developed algorithm in order to determine the VJ and VDJ combinations and CDR3 sequences. Chimerism was determined by microsatellite sequences of DNA of donor and recipient cells. Diversity was calculated for each of TCRA and TCRB using the inverse Simpson’s index.
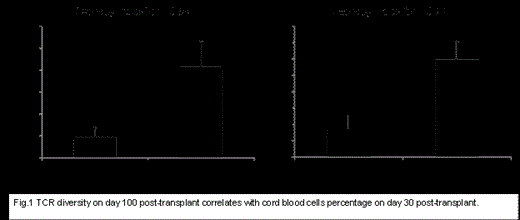
Results:
Several clones found in pre-treatment samples obtained from recipients before transplant persisted at low frequency on days 100 and 365 post-transplant suggesting that these clones have evaded the immuno-suppressive conditioning regimens. In order to correlate the diversity of TCRs with the percentage of cord cells in patients’ blood at different time points, we dichotomized patients into cord present (>5%) and cord absent (≤5%) groups, based on the cord blood percentage in blood on day 30 post haplo-cord transplant and correlated it with diversity on day 100. We found that TCRs of pts with >5% cord cells on day 30 post haplo-cord transplant were significantly more diverse (TCRA; P=0.008) and (TCRB; P=0.01) (Fig.1) on day 100 compared to TCRs in patients with <5% cord cells. Four pts achieved a chimersim of >90% cord cells on day 100 post-transplant. Therefore, we examined the correlation between diversity calculated on day 100 and percentage of persisting haplo-identical cells on the same day. We found,, the diversity of TCRB of pts with persistence of >10% haplo-identical cells were 15.05 compared with 72.95 for those with <10% haplo-identical cells on day 100, (P=0.002); similarly, diversity of TCRA was 17 vs 101.6 (P=0.001) in pts with >10% and <10% haplo cells, respectively. Furthermore, we found several recurring clones that were enriched in recipient blood on days 100 and 365.
In conclusion, we report a novel approach to specifically characterize the TCR repertoire in AML patients receiving haplo-cord transplant. Our approach provides a valuable comprehensive analysis that defines the kinetics of TCRA and TCRB and the changes in CDR3 diversity. This approach, when applied to a larger patient cohort may provide useful information on whether specific TCR clones can be correlated with infectious complications and clinical outcome.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.