Abstract
Hematologic malignancies are more common among older patients. This age group has historically had worse outcomes when compared with younger counterparts. Despite the success of reduced intensity conditioning (RIC), the number of older patients that undergo hematopoietic stem cell transplant (HSCT) after remission is low due to concerns with toxicity and transplant-related mortality (TRM). In this analysis, we aimed to investigate the toxicity and outcome expectations in older patients with hematological malignancies who underwent HSCT at our institution.
We retrospectively analyzed 93 consecutive patients with hematological malignancies aged ≥60 who underwent HSCT in complete remission (CR) between 2009 and 2013. Median age was 63 (range, 60-75) and of 93 patients, 35 (37.6%) were older than age ≥ 65. Most patients had AML (n=81, 87%) followed by CLL (n=6, 6%), ALL (n=5, 5%) and other hematological malignancies (n=1, 1%). Disease status at HSCT was first CR (CR1) in 79 (85%) patients. Hematopoietic cell transplant-comorbidity index (HCT-CI) was 0 in 25 (27%), 1-2 in 25 (27%), 3-4 in 26 (28%) and ≥5 in 13 (14%) patients respectively. Donors were matched related (MRD) in 33 (35%), matched unrelated in 48 (52%), haploidentical in 6 and cord blood (CB) in 6 (6%) patients. Conditioning intensity was myeloablative (MAC) in 68 (73%) and reduced intensity (RIC) in 25 (27%). Graft versus host disease prophylaxis was tacrolimus and methotrexate in 70 (75%) patients. Toxicity after HSCT was monitored real-time and coded per National Cancer Institute (NCI) criteria.
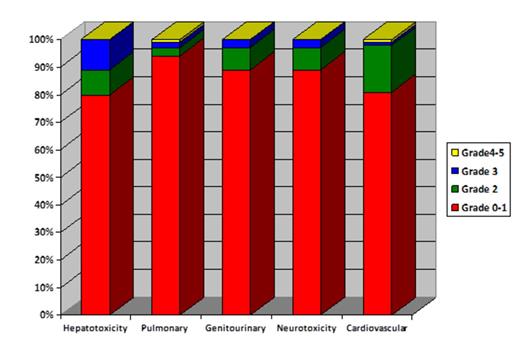
The median follow-up of 54 survivors was 11 months. The median duration of hospital stay was 31 days (interquartile range (IQR, 23-42). Of 93, 12 patients (12.9%) required intensive care unit admission with a median duration of 5.5 days (IQR, 2-9 days). Organ-specific toxicity by day 100 was minimal as presented in Figure 1. Of 93, 4 (4%) patients had definite culture positive fungal pneumonia, 39 (41.9%) had CMV reactivation and 38 (40.8) had at least one episode of documented bacterial infection rather than neutropenic fever by day 100. Transplant related mortality (TRM) at day 100 and 1-year was 4% and 18% as presented in Table 1. The primary cause of TRM at day 100 was infection in 2 and acute GVHD in 1 patients. Similarly at 1-year, the most common causes of TRM were infection in 5, aGVHD in 3, chronic GVHD in 3 and cardiac failure in 1 patients. The 1-year cumulative incidence of progression and progression-free survival (PFS) were 31% and 51% respectively.
In univariate analyses, age ≥ 65 increased TRM with a hazard ratio (HR) 2.1 (p=0.2). The rate of TRM was similar between MUD and MRD recipients with a HR of 1.6 (p=0.5). Haploidentical and CB recipients were not analyzed separately due to their small sample size. Age ≥ 65 also increased the incidence of progression with a HR of 2.1 but that did not reach statistical significance (p=0.1). For PFS, older age was a poor prognostic factor with a HR of 2.1 (p=0.08). Among AML patients, cytogenetics consistent with monosomal karyotype (MK+) increased incidence of progression compared with core binding factor (CBF) and normal cytogenetics (CN) (CBF/CN) patients with a HR of 3.8 (p=0.01). For PFS, older age (HR of 2.1, p=0.04), and MK+ (HR of 3.8,p=0.01) were poor prognostic factors among AML patients. As presented in Figure 2, 39 patients age<65 without MK+ had the best 1-year PFS with 67% while 8 patients age ≥ 65 with MK+ had the worst PFS.
As a summary, our results support the use of HSCT with encouraging tolerability and effectiveness in selected older patients. Improved measures to prevent leukemia relapse are needed in older patients and those with monosomal karyotype.
Transplant outcomes in the study group stratified by disease
| . | Whole cohort n=93 . | AML n=81 . | Other malignancy n=12 . |
|---|---|---|---|
| Day 100 NRM | 4% | 4% | 8% |
| 1-year NRM | 18% | 17% | 22% |
| 1-year progression | 31% | 34% | 8% |
| 1-year PFS | 51% | 48% | 69% |
| 100 days Grade II-IV aGVHD | 33% | 36% | 9% |
| 100 days Grade III-IV aGVHD | 6% | 7% | 0% |
| . | Whole cohort n=93 . | AML n=81 . | Other malignancy n=12 . |
|---|---|---|---|
| Day 100 NRM | 4% | 4% | 8% |
| 1-year NRM | 18% | 17% | 22% |
| 1-year progression | 31% | 34% | 8% |
| 1-year PFS | 51% | 48% | 69% |
| 100 days Grade II-IV aGVHD | 33% | 36% | 9% |
| 100 days Grade III-IV aGVHD | 6% | 7% | 0% |
Progression-free survival in AML patients transplanted in complete remission by age and monosomal karyotype
Progression-free survival in AML patients transplanted in complete remission by age and monosomal karyotype
Andersson:Otsuka Pharmeceuticals: Research Funding, Research funding from Otsuka Pharmeceuticals Other.
Author notes
Asterisk with author names denotes non-ASH members.