Abstract
Introduction: Multiple clinical studies in patients (pts) with newly diagnosed multiple myeloma (NDMM) have shown benefits from continuous lenalidomide (LEN) treatment (Tx), including extended progression-free survival (PFS), time to progression (TTP), and overall survival (OS) (Facon, Blood 2013; Palumbo, NEJM 2012). Dose modifications are commonly utilized by physicians as part of continuous Tx plans to sustain control of disease while managing toxicities. Previous analysis has shown that dose modification of LEN was associated with longer duration of therapy (DOT) relative to non-dose modification, in the real-world setting (Lang, Blood 2013). This study aimed to evaluate whether LEN dose modification is associated with a longer TTP in NDMM pts, utilizing time to next therapy (TTNT) as a proxy measure available from claims data.
Methods: A retrospective study was conducted using a large US medical and pharmacy claims database, covering > 25 million lives annually. NDMM pts were identified with at least 2 outpatient claims or 1 inpatient medical claim associated with a diagnosis of MM (ICD-9-CM code 203.0X), with the first such claim used to define the index date. A minimum of 12 months' pre-index enrollment and 6 months' post-index continuous enrollment between Jan 1 2006 and December 31 2012 was required. The analysis focused on NDMM pts treated with LEN who did not have claims for stem cell transplantation (SCT), to avoid DOT limitations imposed by fixed-length induction Tx. Median DOT and TTNT were compared in NDMM pts with and without LEN dose modifications (dose increase or decrease). Charlson comorbidity scores were determined to compare baseline measures between the groups.
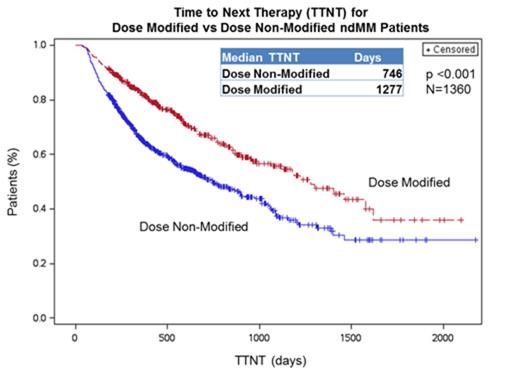
Results: Of the 1,360 pts who met the inclusion criteria, 470 (35%) had LEN dose modifications, including 154 pts with at least one dose increase and 421 pts with at least one dose decrease. The majority of pts had an initial dose of 25mg. The median DOT was 447 days in pts with LEN dose modification and 182 days in those pts with no dose modification (p < 0.001). The median DOT in pts with dose modification was consistent regardless of whether they experienced a dose increase or decrease, with no significant difference. The median TTNT in pts with LEN dose modification was 3.5 yrs compared to 2.0 yrs in pts without dose modification (p < 0.001; Figure 1). Charlson comorbidity scores were similar in pts with and without LEN dose modification.
Conclusions: For non-SCT NDMM patients treated with LEN, dose modifications during their treatment were associated with a doubling of the median therapy duration and a 71% longer median TNTT, compared to pts without LEN dose modification. The DOT and TTNT in pts with dose modification were similar to median values reported for the LEN continuous Tx arm of the FIRST clinical trial (Facon, Blood 2013). This analysis suggests that LEN dose modification in the Tx of NDMM may be an effective tool in the real-world setting for pts to achieve sustained disease Tx and the associated prolonged time to progression, based on the proxy measure of TTNT.
Usmani:Celgene Corporation: Consultancy, Honoraria, Research Funding; Millennium: Consultancy, Honoraria; Onyx: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy; Array BioPharma: Research Funding; Janssen: Research Funding; Pharmacyclics: Research Funding. Off Label Use: Lenalidomide in newly diagnosed multiple myeloma patients. Binder:Celgene Corporation: Employment, Equity Ownership. Milentijevic:Celgene Corporation: Consultancy. Hu:Celgene: Employment. Nagarwala:Celgene Corporation: Employment. Corvino:Genesis Research LLC: Consultancy. Arikian:Genesis Research: Consultancy. Surinach:Genesis Research LLC: Consultancy. Harwin:Celgene Corporation: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.