Abstract
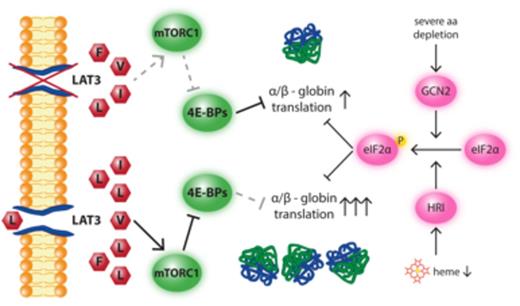
In multicellular organisms, the mechanisms by which diverse cell types acquire distinct amino acids and how cellular function adapts to their availability are fundamental questions in biology. Here, we find that maturing erythroid cells increase L-leucine uptake via transcriptional up-regulation of the L-leucine transporter, LAT3. Inadequate L-leucine uptake by L-leucine starvation or LAT3 inhibition triggers a specific reduction in hemoglobin production in zebrafish embryos and murine erythroid cells via the mTORC1/4E-BP pathway. CRISPR-mediated deletion of 4E-BPs in murine erythroid cells renders them resistant to mTORC1 and LAT3 inhibition, markedly restoring hemoglobinization. Our complementary results demonstrate that globins are direct translational mTORC1 targets during normal development. This pathway is distinct from the previously reported translational regulatory mechanisms mediated by the heme-regulated inhibitor (HRI) kinase or by severe amino acid deprivation via the general control nonderepressible 2 (GCN2) kinase. We propose that, in red cells, mTORC1 serves as a homeostatic sensor coupling hemoglobin production to sufficient L-leucine uptake.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.