Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) can cure SCD but chemotherapy-based conditioning regimens are associated with substantial morbidity and mortality, particularly in adult patients. A chemotherapy-free, non-myeloablative HSCT with alemtuzumab 1mg/kg divided over 5 days followed by total body irradiation (TBI) 300cGy was recently developed at the National Institute of Health and obtained stable mixed chimerism and clinical cure in 90% of SCD patients receiving an HLA and ABO-matched, related HSCT. The same study was replicated in high risk SCD adult patients at the University of Illinois at Chicago.
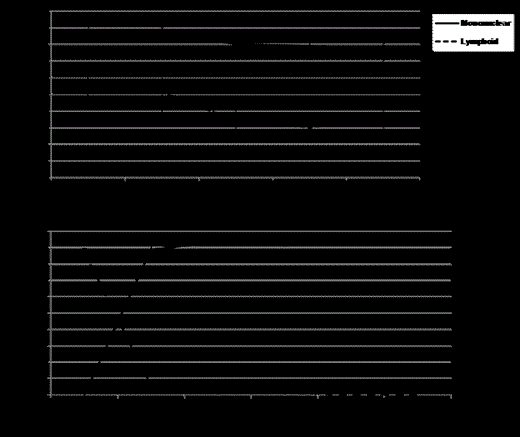
Thirteen patients aged 17 - 40 years were transplanted between November 2011 and June 2014. Indications included ≥3 vaso-occlusive crises (VOC) per year (n=7), stroke (n=4) and >1 episode of acute chest syndrome (ACS) requiring intensive care (n=2). Unmanipulated, G-CSF mobilized peripheral blood cells were collected from HLA-matched, related donors (MRD) with Hb AS (n=4) or Hb AA (n=9). Four patients had red blood cell (RBC) alloimmunization and 2/13 patients received a graft with major ABO incompatibility. The median number of CD34+ cells infused was 8.1 x 106 cells/kg. Of 13 patients, 10 had severe neutropenia for a median duration of 5 days (range: 1 - 14) and 3 patients had a platelet count < 50 x109/L requiring a platelet transfusion. All patients engrafted and are alive with a median follow-up of 337 days (range: 53 – 986). Cytokine levels involved in T-cell regulation (IL-5, IL-10, and IL-13) increased post-alemtuzumab compared to pre-HSCT levels while no differences were observed in IL-2, IL-4, IL-12 or TNF-α. One secondary graft failure occurred in a patient not compliant with sirolimus and no acute or chronic GVHD has been observed. The median donor chimerism at 1 and 6 months was 96% (range: 63 – 98%) and 84% (range: 41 – 96%), respectively, in blood mononuclear cells; and 48% (range: 20 – 97%) and 25% (range: 20 – 48%), respectively, in CD3+ T cells (Figure 1a). Median hemoglobin concentration increased from 8.4 g/dL (range: 6.6 – 12.4 g/dL) to 12.9 g/dL (range: 11.0 – 16.8 g/dL) and the median reticulocyte percentage decreased from 12.1% (range: 4.8 – 19.6%) to 1.9% (range: 1.3 – 6.2%). Changes in hemoglobin fractionation in HSCT recipients from Hb AA donors are shown in Figure 1b. No episodes of ACS or stroke have been observed following hospital discharge after HSCT. Two patients have been tapered off of sirolimus with stable chimerisms, laboratory values, and no repeat episodes of SCD-related complications.
Non-myeloablative HSCT from MRD with alemtuzumab and TBI in SCD adult patients provides a clinical cure in the majority of patients with low toxicity and no transplant-related mortality.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.