Abstract
Background. Mantle cell lymphoma (MCL), which is characterized by the deregulation of cyclin D1 (CCND1), is one of the most common lymphoma subtypes, accounting for 5-10% of all cases. MCL prognosis is often very poor. Several novel non-chemotherapeutic agents have shown promising activity in MCL, but novel agents and in particular new drug combinations are needed to improve patients' outcome. Aberrant changes in histone modifications, DNA methylation and expression levels of non-coding RNA contribute to MCL pathogenesis and represent potential therapeutic targets. Bromodomain and extra-terminal (BET) proteins are epigenetic readers contributing to gene transcription. OTX015 is a bromodomain (BRD) inhibitor that has shown preclinical activity in hematologic and solid tumor models (Gaudio et al, AACR 2014; Noel et al, EORTC-NCI-AACR 2013) as well as promising early results in an ongoing phase I study (Herait et al, AACR 2014; NCT01713582). Here, we present preclinical evidence of OTX015 activity in combination with other targeted drugs in MCL.
Material and methods. MCL cell lines (REC1, MAVER1, UPN1, JeKo1, SP53, Mino, Granta519) were exposed to increasing doses of OTX015 alone or in combination with increasing doses of other drugs, including everolimus, BEZ235, ibrutinib, carfilzomib, pomalidomide, 5-AZA, vorinostat and dexamethasone. The MTT assay was performed after 72h exposure. Real-time PCR and Western blotting were used for RNA and protein expression analyses. Synergy was assessed by the Chou-Talalay combination index (CI) with the Synergy R package: CI<0.3, strong synergy; 0.3-0.9, synergy; 0.9-1.1, additive effect.
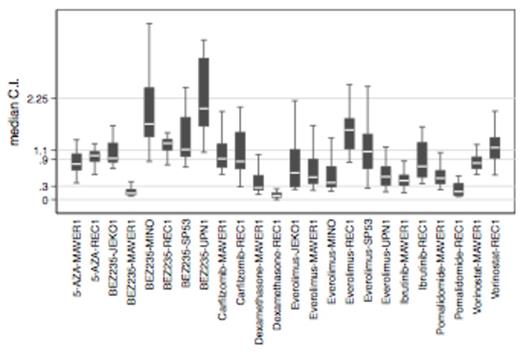
Results. OTX015 showed antiproliferative activity as a single agent in 6/7 MCL cell lines with IC50s < 500 nM. Four MCL cell lines were exposed to DMSO or OTX015 (500 nM and IC50) for 4 and 24h to evaluate expression of CCND1 and other members of signaling pathways known to be impacted by BRD inhibitors. No reduction in CCND1 was observed at the RNA or protein levels after OTX015 exposure. However, MYC was downregulated and the transcriptional regulator HEXIM1 and histone-coding genes were upregulated. In light of reported strong synergy between OTX015 and the mTOR inhibitor everolimus in diffuse large B-cell lymphoma, we evaluated OTX015 combined with everolimus or the dual PI3K/mTOR inhibitor BEZ235 in 6 MCL cell lines (REC1, MAVER1, UPN1, JeKo1, SP53, Mino). In addition, combinations of OTX015 with the BTK-inhibitor ibrutinib, the proteasome inhibitor carfilzomib, the immunomodulator pomalidomide, the demethylating agent 5-AZA, the HDAC-inhibitor vorinostat, and the glucocorticoid dexamethasone were assessed in REC1 and MAVER1. Combinations of OTX015 with everolimus, pomalidomide, dexamethasone, and ibrutinib showed the strongest activity (Fig 1). Strong synergy between BEZ235 and OTX015 was only seen in the ibrutinib-resistant cell line MAVER1.
Conclusions. OTX015 showed preclinical activity as a single agent in MCL cells. The mechanism of action does not appear to involve CCDN1 down-regulation and gene expression profiling studies will be needed to identify the involved pathways. OTX015 had additive or synergistic activity with several targeted compounds in multiple MCL cell lines, identifying combinations that may merit further investigation in the preclinical and clinical settings.
Chou-Talalay analysis of OTX015 combinations in MCL cell lines. Y-axis: CI<0.3, strong synergy; 0.3-0.9, synergy; 0.9-1.1, additive effect; > 2.25, antagonism. Outliers were excluded. C.I., Combination Index.
Chou-Talalay analysis of OTX015 combinations in MCL cell lines. Y-axis: CI<0.3, strong synergy; 0.3-0.9, synergy; 0.9-1.1, additive effect; > 2.25, antagonism. Outliers were excluded. C.I., Combination Index.
Stathis:Oncoethix SA: Consultancy, Research Funding. Riveiro:Oncoethix SA: Consultancy, Research Funding; Oncology Therapeutic Development: Employment. Bertoni:Oncoethix SA: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.