Abstract
As cure rates for acute lymphoblastic leukemia (ALL) have increased, toxic complications of chemotherapy have also increased. The incidence of osteonecrosis is high – 54% of children had asymptomatic osteonecrosis, and 18% experienced symptomatic osteonecrosis in one prospective study; osteonecrosis was positively associated with plasma dexamethasone exposure (Kawedia et al. Blood 2011 PMID: 21148812). Osteonecrosis is also more common after continuous than discontinuous dosing of dexamethasone in children (Mattano et al. JCO 2012 PMID: 22901620, Seibel et al. Blood 2008 PMID: 18039957). Many ALL clinical trials now include a discontinuous glucocorticoid schedule, in which patients receive dexamethasone daily for one week, none the second week, then daily for the third week, in an attempt to reduce the rate of osteonecrosis.
Using a murine model of osteonecrosis, our group previously found that mice treated with a discontinuous regimen of dexamethasone developed less osteonecrosis than those treated with a continuous regimen (Yang 2008 PMID: 18683891), consistent with clinical data. However, it is not known whether the discontinuous schedule may also compromise the cure rate. Our goal was to use murine models of ALL to determine if there was a difference in antileukemic efficacy of the continuous versus discontinuous dexamethasone regimens.
Each of six patient samples of various ALL subtypes was engrafted into immunocompromised NSG mice, by intravenous injection of 1-5 million cells. In vitro sensitivity to dexamethasone was assessed at the time of diagnosis, and all samples were sensitive to nanomolar concentrations. The samples were passaged serially: they were transduced with a lentiviral vector containing YFP and luciferase prior to the second passage, sorted for YFP+ cells prior to the third passage, and tested for in vivo efficacy of continuous (cont) and discontinuous (disc) dexamethasone on the fourth and/or fifth passage. Dexamethasone was given in the drinking water, either half the week at 8 mg/L (Disc dex) or continuously at 4 mg/L (Cont dex) starting 7-28 days from ALL injection. All mice received antibiotic prophylaxis in the drinking water. Plasma concentrations of dexamethasone in mice on treatment were similar to plasma levels (20 – 200 nM) achieved in patients with ALL (Yang et al JCO PMID: 18421047).
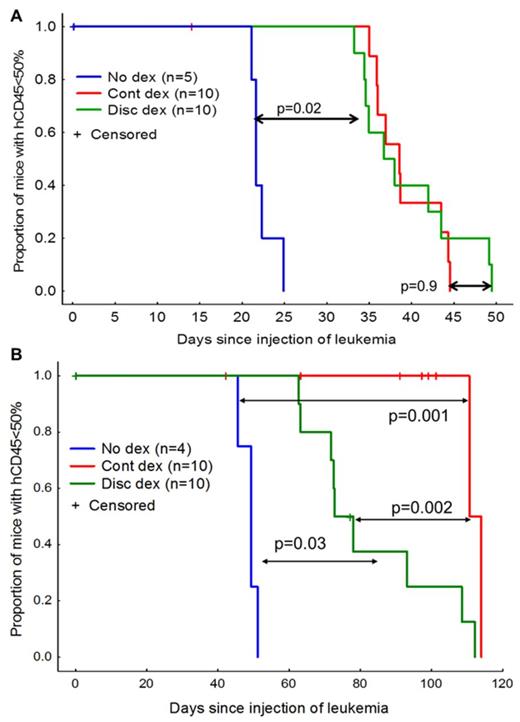
For all ALL samples engrafted, there was improved leukemia-free survival (LFS) with dexamethasone treatment compared to no dexamethasone treatment (median LFS +4 to +129 days relative to no dexamethasone, log-rank p=0.02 to p=0.001). For five of the six samples, there were no differences in LFS between the continuous and discontinuous regimens (median LFS differed by 1 – 15 days, log-rank p=0.14 to 0.9). For one of the six samples, the continuous dexamethasone was more effective (median LFS of 111 vs 77 days, log-rank p=0.002) than the discontinuous schedule. In a second experiment with the same sample, when all the mice were sacrificed on the same day from start of therapy, ALL burden as assessed by ventral luminescence (Kruskal-Wallis p=0.004) and by spleen size (Kruskal-Wallis p=0.006) was lower in the continuous dexamethasone group vs the discontinuous dexamethasone group.
Thus far, these data indicate that for most pediatric ALL, discontinuous dexamethasone is likely to be as effective as continuous dexamethasone; however, it is possible that for some ALL cases, there may be differences in ALL efficacy between the continuous and discontinuous dexamethasone regimens.
Antileukemic efficacy of continuous (cont) vs discontinuous (disc) dexamethasone for two xenografted patient ALL samples. A, Representative data from mice engrafted with one of the 5 samples that showed equal efficacy with the two dexamethasone regimens (p=0.9). Mice were treated from days 7 – 50. Survival was significantly longer with both dexamethasone regimens than with no dexamethasone (n=5, p=0.02). B, Mice engrafted with a single patient sample show superior anti-leukemic efficacy with cont dexamethasone vs disc dexamethasone (p=0.002). Mice were treated from days 21-91.
Antileukemic efficacy of continuous (cont) vs discontinuous (disc) dexamethasone for two xenografted patient ALL samples. A, Representative data from mice engrafted with one of the 5 samples that showed equal efficacy with the two dexamethasone regimens (p=0.9). Mice were treated from days 7 – 50. Survival was significantly longer with both dexamethasone regimens than with no dexamethasone (n=5, p=0.02). B, Mice engrafted with a single patient sample show superior anti-leukemic efficacy with cont dexamethasone vs disc dexamethasone (p=0.002). Mice were treated from days 21-91.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.