Abstract
Introduction: Despite the improvement of outcome of aggressive B-cell lymphomas in the rituximab treatment era, central nervous system (CNS) relapse continues to pose a significant management problem. It remains a challenge to select patients (pts) in whom specific diagnostic procedures to identify CNS disease at diagnosis should be performed and to target a high risk group in whom a CNS prophylaxis strategy is warranted. The German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) recently proposed a new prognostic model incorporating the 5 IPI factors (age > 60 y, LDH > N, stage 3 or 4, extranodal (EN) sites > 1) in addition to kidney/adrenal gland involvement to predict the risk of secondary CNS disease in pts with aggressive B-cell lymphoma. This model effectively stratified patients into 3 risk groups: low risk (0-1 factors, 2 y CNS relapse risk .6% (95% CI 0.0-1.2); intermediate risk (2-3 factors, 2 y CNS relapse risk 3.4 %( 95% CI 2.2-4.6)) and high risk (4-6 factors, 2 y CNS relapse risk of 10.2% (95% CI 6.3-14.1)) (Schmitz et al. Hematol. Oncol. 2013: 31, 047a). Herein, we sought to validate this model in an independent cohort of DLBCL treated with R-CHOP chemotherapy at the British Columbia Cancer Agency (BCCA).
Methods: The DSHNHL dataset on which the model was initially developed was comprised of 2164 patients with aggressive B-cell lymphomas (n= 1735, 80.2% diffuse large B-cell lymphoma (DLBCL)), 18-80 years of age who were treated with rituximab with (CHO(E) P-like chemotherapy on prospective studies. The BCCA Lymphoid Cancer Database was screened to identify all patients with DLBCL treated with curative intent R-CHOP chemotherapy.
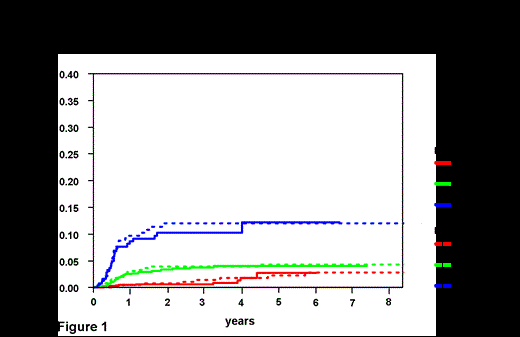
Results: In total, 1597 patients were diagnosed with DLBCL at the BCCA and received at least one cycle of curative intent R-CHOP chemotherapy. The median follow-up for living patients was 4.2 years. Pts in the BCCA population-based DLBCL cohort were more likely to have poor risk features including PS >1, advanced age and a high IPI score (Table 1). Applying the 6 factor model, very similar risk groups were identified: low risk (0-1 factors 2 year CNS relapse risk .8% (95% CI 0.0-1.6%).; intermediate risk (2-3 factors 2 year CNS relapse risk 3.9% (95% CI 2.3-5.5%); and high risk (4-6 factors 2 y CNS relapse risk 12% (95% CI 7.9-16.1%) (Figure 1). The median time to CNS relapse was 6.7 months from the time of diagnosis in the BCCA group and was 7.2 months in the DSHNHL group highlighting that this event typically occurs early in the disease course. In both datasets kidney/adrenal involvement was highly associated with CNS relapse (2 year CNS risk BCCA 33%; 14% DSHNHL), the difference likely reflecting the higher risk pts in the BCCA population-based setting.
Conclusions: We have validated the proposed DSHNHL prognostic model for CNS relapse in an independent dataset. The model identifies a high risk group in which diagnostic procedures to rule out CNS disease are highly recommended at diagnosis including MRI head, and cerebrospinal fluid analysis by cytology and flow cytometry and consideration of CNS-directed therapies. Kidney/adrenal involvement is consistently associated with a high risk of CNS relapse in the rituximab treatment era for which CNS prophylaxis should be incorporated into front-line therapy.
| Clinical factor . | BCCA N=1597 . | DSHNHL N=2164 . |
|---|---|---|
| Age > 60 years * Median age | 1035 (65%) 65 years (16-94) | 974 (45%) 58 years (18-80) |
| Median follow-up | 4.6 years | 2.9 years |
| Male sex | 915 (57%) | 1244 (57.5%) |
| PS > 1* | 584 (37%) | 247 (11%) |
| Elevated LDH | 1147 (53.0%) | 737 (49.0%) |
| EN > 1 | 396 (25%) | 479 (22%) |
| Stage 3 or 4 | 916 (57%) | 1148 (53%) |
| IPI * 0,1 2 3 4,5 | 463 (31%) 359 (24%) 350 (23%) 329 (22%) | 1009 (47%) 523 (24%) 398 (18%) 231 (11%) |
| Bulky disease > 7cm | 636 (41%) | 1027 (47.5%) |
| Clinical factor . | BCCA N=1597 . | DSHNHL N=2164 . |
|---|---|---|
| Age > 60 years * Median age | 1035 (65%) 65 years (16-94) | 974 (45%) 58 years (18-80) |
| Median follow-up | 4.6 years | 2.9 years |
| Male sex | 915 (57%) | 1244 (57.5%) |
| PS > 1* | 584 (37%) | 247 (11%) |
| Elevated LDH | 1147 (53.0%) | 737 (49.0%) |
| EN > 1 | 396 (25%) | 479 (22%) |
| Stage 3 or 4 | 916 (57%) | 1148 (53%) |
| IPI * 0,1 2 3 4,5 | 463 (31%) 359 (24%) 350 (23%) 329 (22%) | 1009 (47%) 523 (24%) 398 (18%) 231 (11%) |
| Bulky disease > 7cm | 636 (41%) | 1027 (47.5%) |
P< 0.05
Savage:F Hoffmann-La Roche: Other. Villa:F Hoffmann-La Roche: Other. Sehn:Roche: Research Funding. Pfreundschuh:Roche: Advisory Board Other, Research Funding. Gascoyne:Hoffman La-Roche: Research Funding. Connors:Seattle Genetics, Inc.: Research Funding; Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract