Abstract
Introduction: Myelodysplastic syndromes (MDS) are clonal stem cell disorders associated with bone marrow failure. Allogeneic stem cell transplantation (alloSCT) is currently the only curative therapy option for patients with MDS. However, on the one hand, most MDS patients are older than 60 years and non-relapse mortality (NRM) remains a significant problem. Therefore, only patients with higher risk MDS according to IPSS (intermediate-2 or higher) appear to profit from alloSCT. On the other hand, higher risk MDS patients have increased relapse rates following alloSCT. In order to optimize the application of alloSCT in this disease, both relapse and NRM rates need to be reduced. We have recently provided evidence that weight loss and minor metabolic changes prior to alloSCT were able to predict relapse and death of acute myeloid leukemia patients using data from two independent patient cohorts
This retrospective study investigated the influence of pre-transplant weight loss on clinical outcome of MDS patients after alloSCT in three independent cohorts.
Patients and methods: A total of 110 patients (59% male) with a median age of 53 years were included into the analysis. Patients have been diagnosed with MDS according to WHO criteria and received an alloSCT between 2000 and 2012 in three different German referral centers (Heidelberg, Dresden and Berlin). Patient data was retrospectively collected by medical chart review according to the declaration of Helsinki and with approval of the local Ethics committees. Weight data were raised from medical records by three independent researchers in three independent institutions. Weight loss (expressed in percent) was calculated on the basis of recorded weight data at the time of alloSCT and the maximum weight in the time period of 3-6 months prior to alloSCT. The MDS WHO subtype was RA(RS)/RCMD in 31 patients (28%), RAEB1 in 30 patients (27%) and RAEB2 in 49 patients (45%). According to IPSS 31%, 47% and 22% of the patients were in the risk groups intermediate-1, intermediate-2 and high, respectively. The majority of the patients (n=71, 65%) was previously untreated. Twenty-four patients (22%) and 14 patients (13%) received hypomethylating agents and classic chemotherapy prior to alloSCT, respectively. Thirty-one patients (28%) received transplants from related donors (RD), 58 patients (53%) from matched unrelated donors (MUD) and 21 (19%) from mismatched unrelated donors (MMUD). Ninety-three patients (85%) received reduced intensity conditioning (RIC) and 17 patients (15%) received standard myeloablative conditioning (MAC). Survival times were measured from date of alloSCT. Overall survival (OS), relapse-free survival (RFS), NRM and were calculated from date of alloSCT to the appropriate endpoint. Cox regression analysis was applied for OS, NRM and RFS. Relapse and NRM were considered as competing risks. As confounding prognostic factors we included weight loss (continuous), IPSS score, pretreatment, donor type, sex mismatch with the donor and type of conditioning.
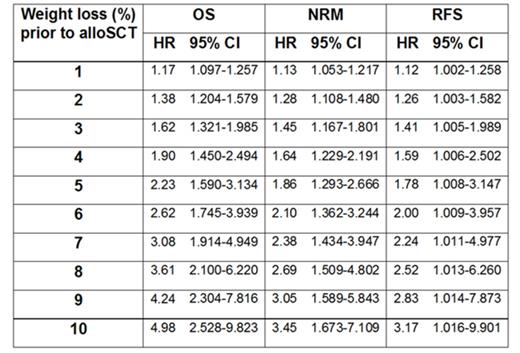
Results: Median follow-up time was 36 months. For both endpoints OS and NRM multivariate analysis revealed a significant interaction between weight loss and donor type (p<0.01). In Cox regression analysis with the endpoint RFS only weight loss (p=0.05) was associated with significantly shorter RFS. The prognostic effect of pre-transplant weight loss on OS, NRM and RFS and the increasing hazard ratios (HR) with corresponding confidence intervals (CI) for different percentages of weight loss are given in Table 1 (multivariate Cox regression). In a mixed effect model with weight loss as outcome age, recipient sex and pretreatment had no significant impact on weight loss. However, there was a trend towards increased weight loss in patients with high risk MDS according to IPSS (p=0.07).
Conclusion: Our retrospective study suggests that in MDS patients pre-transplant weight loss is associated with both higher disease recurrence and higher NRM resulting in survival disadvantage after alloSCT. Prospective studies addressing pre-transplant nutritional interventions in order to improve the outcome of MDS patients are highly warranted.
Table 1.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.