Abstract
Background: Multiple myeloma (MM) in relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a challenging situation because of particular aggressive disease, frail patients and a specific immune context. Our previous data suggested that lenalidomide salvage therapy could lead to an overall response rate (ORR) of 83%, and a progression free survival (PFS) of 18 months. However, this was achieved at the cost of 25% incidence of de novo acute graft versus host disease (aGVHD). On the other hand, bortezomib has both anti- myeloma activity and a potential ability to modulate GVHD. With this background, this study aimed to evaluate the efficacy, tolerance and immunomodulatory effect of bortezomib salvage therapy post allo-HSCT.
Patients: This retrospective study included 97 patients (15 SFGM-TC centers) with MM who relapsed after allo-HSCT and received bortezomib as salvage therapy any time post allo-HSCT. Median age at allo-HSCT was 50 years (range: 29-64). Most of the patients had high-risk MM (72% with poor-risk cytogenetics) and received a median of 2 lines (range: 1-5) before allo-HSCT. Previous autologous HSCT was performed in 95% of cases (2 autologous HSCT in 25% of cases). Allo-HSCT was mainly performed after a reduced intensity conditioning (94%) from a sibling donor (67%). The median time of relapse/disease progression after allo-HSCT was 15 months (range, 1-84). Bortezomib treatment started at a median of 24 months (range: 1-97) after allo-HSCT (first line salvage therapy in 53% of cases).
Results: Patients received a median of 4 cycles of bortezomib (range, 0.5-13), combined with dexamethasone in 66% of cases. ORR was 58% (16% complete response, 20% very good partial response, 22% partial response), with the best response being achieved after a median of 4 cycles (range: 1-7). ORR was independent of cytogenetic status at diagnosis (p=0.19), dexamethasone use (p= 0.39) and bortezomib introduction (first line therapy post relapse after allo-HSCT versus beyond first line) (p=0.68).
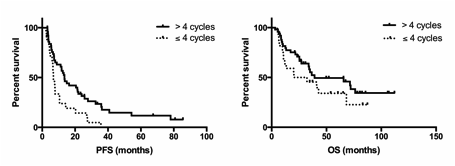
With a median follow-up of 34 months (range: 1-112) after bortezomib administration, the median PFS was 7 months (range: 1-86) and the median overall survival (OS) was 27 months (range: 1-112). Dexamethasone association, cytogenetic status and timing of bortezomib introduction did not impact PFS or OS. Excluding patients who relapsed before the 4th cycle of treatment (n=25), we show that prolonging bortezomib administration > 4 cycles (n=62), independently of being in response at the 4th cycle, improved PFS from 6.8 to 13.5 months (Hazard ratio (HR)=0.4, 95%CI: 0.2-0.7, p=0.01) while the statistical significance wasn't reached for OS (increase from 26.2 to 39.1 months; HR=0.6, 95%CI: 0.3-1.2, p=0.15) (Figure 1).
During bortezomib therapy, no occurrence of acute GVHD was observed, whereas 7 patients (7%) developed chronic GVHD (cGVHD) (4 de novo cases and 3 worsening of pre-existing cGVHD). On the other hand, 7 cases of GVHD (6 chronic and 1 acute) improved during bortezomib therapy. Bortezomib was administered intravenously for all patients. Usual grade 3/4 hematological (21% thrombocytopenia), neurological (16%), fatigue (16%) and infectious (9%) side effects were observed. Toxicity led to treatment discontinuation in 27% of patients. Most of the side effects (96%) were reversible without any death due to treatment toxicity.
Conclusion: Bortezomib is safe and effective in case of MM patients in relapse post allo-HSCT. ORR was about 58% with a median PFS of 7 months. A prolonged administration (> 4 cycles) may improve PFS. The efficacy of bortezomib in this situation does not seem to be linked to an enhanced graft versus myeloma effect since we observed both improvement and worsening of GVHD after the introduction of this treatment. These data contrast to our previous report on lenalidomide and our results will be discussed in order to identify the best treatment option in this particular difficult medical situation and to lead to further prospective studies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.