Abstract
Introduction: Daily plasma exchange (PE) has become a definitive first-line treatment for acquired thrombotic thrombocytopenic purpura (TTP). However, it remains to be controversial whether platelet transfusion is harmful or not for patients with acquired TTP during initial treatment. Some articles showed that platelet transfusion was considered as hazardous because platelet transfusion might generate widespread fresh platelet aggregates in circulation, but others reported that the mortality rate was not different between patients with and without platelet transfusion. Herein, we conducted a retrospective analysis of a large cohort of patients with acquired idiopathic TTP (ai-TTP) in Japan evaluating whether platelet transfusion was associated with unfavorable outcomes.
Patients and Methods: Our laboratory has been functioning as a nationwide referral center for thrombotic microangiopathies (TMAs) in Japan. We collected a large dataset of medical information on 1211 patients with TMA from March 2000 to December 2013. Among them, 263 were ai-TTP patients with severe deficiency (<10% of normal controls) of ADAMTS13 activity (ADAMTS13:AC), with positive anti-ADAMTS13 inhibitors. These ai-TTP patients were retrospectively analyzed in detail using reported medical records that contained detailed clinical data and outcome information. ADAMTS13:AC was determined by classic von Willebrand factor multimer (VWF) assay. These results were available within 4 to 7 days in the initial period (March 2000 to March 2005, n=91). Subsequently, with use of a chromogenic ADAMTS13-act-ELISA, reporting occurred within 2 days of obtaining a plasma sample (April 2005 to December 2013, n=172). All plasma samples collected before March 2005 were re-examined by act-ELISA.
Results: In 263 ai-TTP patients, 48 patients received platelet transfusions during the initial treatment and 215 did not. Factors associated with increased mortality included age greater than 60 years and presentation with central nervous system (CNS) dysfunction (p<0.05 for each), but not use of platelet transfusions [22.9% (11/48) versus 17.7% (38/215), respectively].
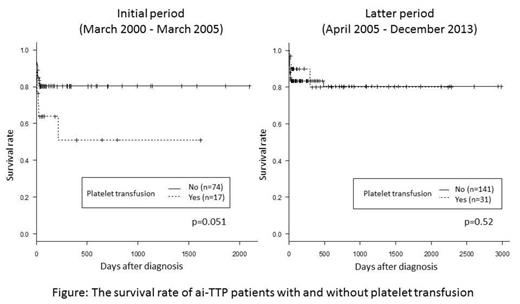
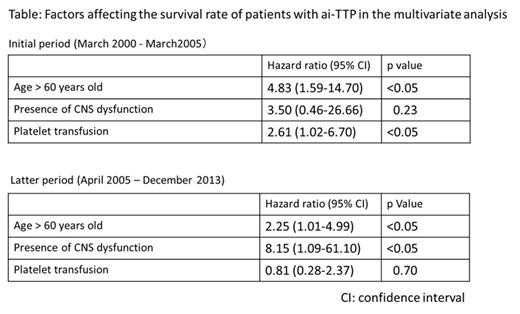
Next, we analyzed the mortality rate in 2 groups categorized by method of ADAMTS13 activity assay. In the initial period using classic VWF assay, mortality of patients with platelet transfusion tended to be higher versus those without platelet transfusions (p=0.051, Log-rank test) as shown in Figure left (Kaplan-Meier survival curve). In contrast, in the latter period using the more rapid act-ELISA, no differences in mortality of patients with versus without platelet transfusions was noted (p=0.52, Figure right). To evaluate whether platelet transfusion is a significant hazard for patients with ai-TTP, Cox-proportional-hazards regression analysis was performed using 5 variables (age, sex, rituximab use, presence of CNS dysfunction, and platelet transfusion). Older age and use of platelet transfusion were independently associated with mortality in the initial period, and age and presence of CNS dysfunction were identified as unfavorable factors in the latter period (Table).
Conclusions: Our results clearly indicated that platelet transfusion was harmful in ai-TTP patients with severe deficiency of ADAMTS13:AC before 2005 (when a quick ADAMTS13:AC assay was unavailable). After 2005 (when a quick ADAMTS13:AC assay available), the survival rate of ai-TTP patients with platelet transfusions became almost indistinguishable from those without. This result may indicate that persistent PE after platelet transfusion together with confirmation of severe ADAMTS13:AC reduces risk for aggravated platelet thrombi formation in microvasculatures of patients. However, use of platelet transfusion before PE may lead to serious thrombotic complications in heart and brain, a complication that should be avoided.
Matsumoto:Alfresa Pharma Corporation: Patents & Royalties. Fujimura:Alfresa Pharma Corporation: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.