Abstract
Background and Objective
Anticoagulants play a pivotal role in the treatment of thromboembolic disorders. Haemorrhage in surgical patients receiving anticoagulants is a major concern. Antidotes are administered to counteract anticoagulation and to restore normal hemostasis. To date, protamine sulphate (PS), a cationic polypeptide is the only clinically approved antidote for unfractionated heparin. PS has toxic side effects and limitations. Inability of PS to completely reverse low molecular weight heparins and fondaparinux is due to its low binding affinity to these drugs. However, PS interacts with coagulation proteins such as fibrinogen to form aggregates which leads to cardiovascular adverse effects. Recently, we developed a synthetic universal heparin reversal agent (UHRA) with high binding affinity to heparins. In vivo studies revealed that UHRA completely reverse the activity of all clinical available parenteral anticoagulants and is nontoxic. This study aims to demonstrate the nontoxic nature of UHRA by assessing its influence on fibrinogen, fibrin clot architecture, plasma clotting and clot lysis.
Methods
UHRA was developed by incorporating tertiary amine based heparin binding groups on a dendritic hyperbranched polyglycerol scaffold and capping it with methoxy polyethylene glycol chains. Recalcification and tissue factor (TF) initiated turbidimetric plasma clotting assays was performed to understand the impact of UHRA on coagulation system. The interaction of UHRA on fibrinogen was investigated by fibrinogen aggregation assay, fibrin polymerization assay and by spectroscopic analysis (fluorescence and circular dichroism (CD)). The influence of UHRA on fibrin clot architecture was evaluated by scanning electron microscopy (SEM).The anticoagulant neutralization (heparins) by UHRA was studied by fluorogenic thrombin generation assay (TGA) in human platelet-rich plasma (PRP). The lysis of TF-induced plasma clot containing UHRA or PS exposed to exogenous tissue plasminogen activator (t-PA) was studied by turbidimetric assay.
Results and discussion
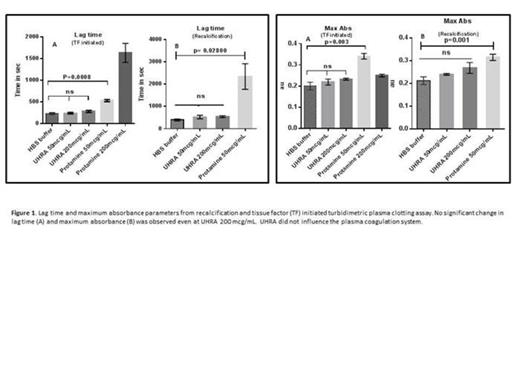
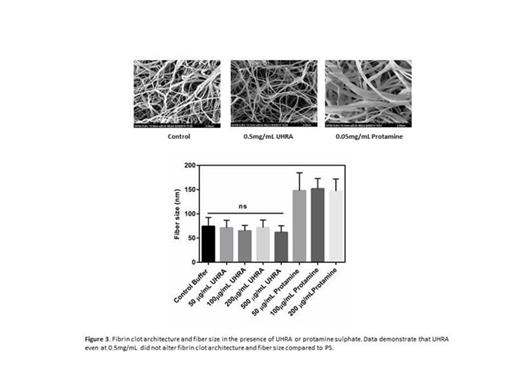
Results from the plasma clotting assays showed that UHRA did not alter the clotting parameters compared to PS (TF initiated lag time and maximum absorbance, control vs UHRA 200 mcg/mL, p=0.21 and 0.16, respectively; lag time and maximum absorbance in recalcification, control vs UHRA 200mcg/mL, p=0.08 and 0.13, respectively) suggesting that UHRA has no effect on coagulation system at the concentration studied (Figure 1). Unlike protamine, the fibrinogen aggregation and fibrin polymerization assay was not influenced by UHRA over a broad range of concentrations from 0.05mg/mL to 1mg/mL. Together with tryptophan fluorescence quenching measurements (Figure 2) and fibrinogen secondary structure measurements corroborates that UHRA is not interacting with fibrinogen. The results are quite different from PS and other synthetic cationic polymers which interact with fibrinogen eliciting aggregation and conformational changes. Fibrin clots generated in presence of UHRA (even at 0.5 mg/mL) showed similar structure and fiber size remains same as normal fibrin clot (control vs UHRA 0.5 mg/mL clot, p= 0.12) (Figure 3). On the other hand, fibrin clots formed in the presence of 0.05mg/mL PS (clinical dose) increased the fiber size and changed the clot structure dramatically (control vs PS 0.05mg/mL clot, p< 0.0001). Our plasma clot lysis studies in the presence of exogenous t-PA demonstrate that UHRA did not enhance clot degradation unlike protamine. UHRA restored thrombin levels in anticoagulated PRP (heparinized) demonstrating the efficacy.
Conclusion and significance
Our studies demonstrate that universal heparin antidote, UHRA, has negligible impact on fibrinogen, fibrin polymerization, clot structure, clot degradation and the coagulation system revealing their excellent hemocompatibility compared to protamine. Our results support the fact that UHRA could be an ideal antidote to restore hemostasis following invasive surgical procedures and to address bleeding complications by heparin based anticoagulants.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.