Abstract
Introduction: XPO1 (CRM1, Exportin 1) is the sole transporter of many tumor suppressor proteins (including MYC, BCL2, BCL6, BTK, IkB) and is elevated in non-Hodgkin Lymphoma. Selinexor (Sel, KPT-330) is an oral covalent inhibitor of XPO1, the first clinical molecule of the Selective Inhibitors of Nuclear Export drug class. The phase I clinical trial of Sel in hematologic malignancies showed promising early single-agent efficacy with modest toxicity in relapsed Diffuse Large B-cell Lymphoma (DLBCL, Gutierrez et al, ASCO 2014). DLBCL, the most common lymphoid malignancy, is currently cured in only 10% of relapsed patients, and consists of 2 subtypes based on putative cell of origin (COO): activated B-cell (ABC) and germinal center B-cell (GCB). We performed preclinical studies of Sel, modeling its single-agent efficacy in frontline and relapsed DLBCL and its potential synergy with other clinically relevant therapeutics.
Long-term follow-up data on patients(pts) treated with Radiolabeled antibody 90-yttrium-Ibritumomab Tiuxetan (RIT) are necessary to define the role of RIT-therapy (RIT-t) in current clinical practice. There are few reports about the use of RIT outside clinical trials.
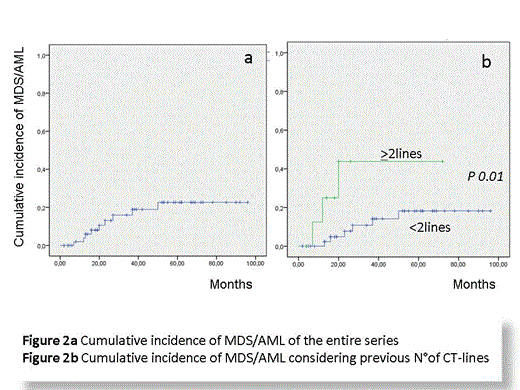
Outcome and toxicity of radioimmunotherapy remain a matter of concern. Incidence of MDS/AML reported after RIT-t as consolidation in front line varies from 3 to 4.3% (Czuczman et al, JCO 2007; Morschhauser et al, JCO 2012). A recent study with RIT and autologous stem cell transplantation (ASCT) as front line therapy in mantle cell lymphoma reported 20% incidence of secondary malignancies, (Arranz et al, Haemat 2013).
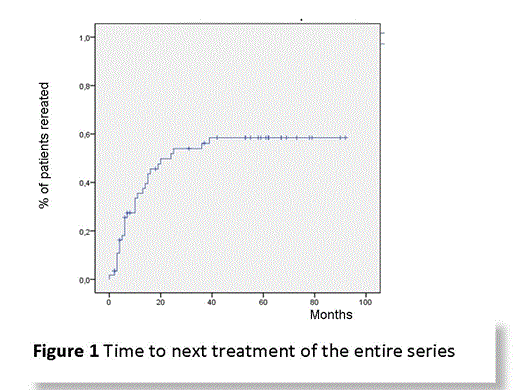
So the aim of this study was to analyze the outcome of pts treated with RIT-t in clinical practice and to assess the incidence of secondary neoplasia particularly MDS/AML.
We included in this retrospective analysis all pts with Diffuse B Large Cell Lymphoma (DBLCL), Follicular Lymphoma (FL) and Marginal Zone lymphoma (MZL), treated with RIT-t over the last decade at our Institution. We adopted the standard RIT therapeutic regimen: infusion of 250 mg/m2 rituximab on day 1, a second 250 mg/m2 dose on day 7, followed by dose of RIT (0.4 mCi/kg), administered 4 h following rituximab infusion. Response assessment was made as recommended by International Workshop criteria. Survival analysis was performed with Kaplan-Meier method and univariate analysis with Log-Rank test with a significance at 0.05.
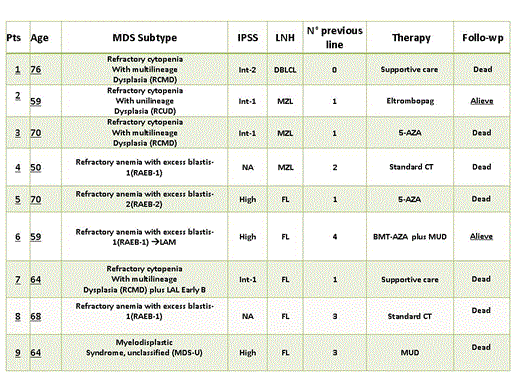
Between March 2006 and January 2014 61 pts underwent RIT-t, of these 57 had complete data and were considered for analysis. There were 16 DBLCL, 29 Follicular Lymphoma and 12 MZL. Twentythree were male, 34 female. Median age at diagnosis was 62 years(yrs) (range 36; 79), 66yrs (range 39;79) at RIT-t. Thirteen pts (all DBLCL) received RIT-t as front line consolidation. Forty-four pts (3 DBLCL, 12 MZL, 29 FL) underwent RIT-t after ≥1 therapy line: one prior line was received by 15pts, 2 by 19, 3 by 8 and 4 by 2. Before RIT-t 24pts were in relapse (2DBLCL, 2MZL, 20FL), 9 were refractory (2 MZL, 7 FL), 7 in partial response (1 DBLCL, 5 MZL, 1FL) while 4 were in complete remission (2 MZL, 2FL).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.