Abstract
Background: Ponatinib is a potent oral pan–BCR-ABL tyrosine kinase inhibitor (TKI) active against native and mutated forms of BCR-ABL, and is approved for patients with refractory CML and Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia and those with the T315I mutant. Long-term follow-up of the anti-leukemic activity and safety of ponatinib in patients with CML or Ph+ ALL in this phase 1 clinical trial is reported.
Methods: Patients (N=81) with resistant/refractory hematologic malignancies were enrolled in this ongoing, open-label, dose-escalation, phase 1 study (NCT00660920). Ponatinib was dosed once daily (2 mg-60 mg). Intra-patient dose escalation was permitted. The 43 patients who had CP-CML are the focus of this analysis (data as of 6 Jan 2014). Median follow-up for CP-CML patients was 42.5 (1.7-59.1) months.
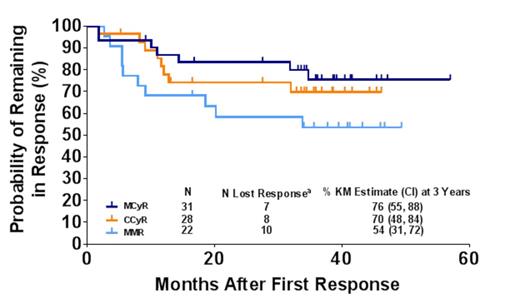
Results: The median age of patients was 55 years; median time since diagnosis was 6.6 years. Patients were heavily pretreated (61% received ≥3 prior TKIs; 37% received 2 prior TKIs). At baseline, 63% of patients had BCR-ABL mutations (28% with T315I). At the time of analysis, 56% of patients remained on study. Significant anti-leukemic activity was observed: major cytogenetic response (MCyR), complete cytogenetic response (CCyR), major molecular response (MMR) and molecular response 4 (MR4) rates were 72%, 65%, 51%, and 40% respectively; 76% of patients with MCyR and 54% with MMR are estimated (Kaplan-Meier [KM]) to maintain response for at least 4 years (3-year KM estimates: 76% MCyR, 70% CCyR, 54% MMR; Figure 1 ). 15 patients started at a dose of 30 mg or below, and 10 of these patients (67%) achieved MCyR; all were receiving a dose of 30 mg or below at time of response (Table 1). Of 28 patients with CCyR, 22 remained on study (16 with continuous CCyR); of 22 patients with MMR, 19 remained on study (11 with continuous MMR). Adverse events (AEs, 23%) and progression (9%) were the most common reasons for discontinuation. Of the 10 patients that discontinued due to AEs, 5 were in MCyR and, of those, 1 was in MMR. The most common treatment-emergent AEs were rash (63%), fatigue (61%), abdominal pain (58%), headache (56%), and arthralgia (54%). The most common treatment-emergent AEs occurring after 1 year of therapy were fatigue (35%), hypertension (31%), and abdominal pain (30%). Treatment-emergent arterial thrombotic events (AE [SAE]) were observed in 37% [28%] of patients (composite of cardiovascular 28% [19%], cerebrovascular 9% [7%], and peripheral vascular 12% [7%] events), and venous thromboembolic AEs were observed in 5% [no SAEs] of patients. Updated data will be presented.
Conclusions: With a median follow-up of 42.5 months in CP-CML patients (maximum follow-up, 59.1 months), ponatinib continues to provide benefit to heavily pretreated patients with limited treatment options. Substantial and durable responses were observed with ponatinib, and responses were observed in patients treated with doses at or below 30 mg. The most common treatment-emergent AEs occurring after one year of therapy were similar to the overall AE profile, albeit with lower incidence rates. Risk and benefit considerations should be evaluated when utilizing ponatinib in this patient population.
aLoss of response is defined as a single time point at which the criteria for response are not met.
Ponatinib Response Rate for CP-CML Patients by Dose
| Starting Dose . | MCyR . | MMR . | ||
|---|---|---|---|---|
| N (%) | Dose intensity*, Median (min, max) | N (%) | Dose intensity*, Median (min, max) | |
| 4 mg, N=3 | 2 (67) | 3.7 (3.5, 3.9) | 1 (33) | 14.3 (14.3, 14.3) |
| 15 mg, N=7 | 5 (71) | 14.8 (14.7, 23.7) | 4 (57) | 15.0 (14.7, 36.6) |
| 30 mg, N=5 | 3 (60) | 27.4 (10.6, 29.6) | 1 (20) | 29.9 (29.9, 29.9) |
| ≤30 mg, N=15 | 10 (67) | 14.8 (3.5, 29.6) | 6 (40) | 15.0 (14.3, 36.6) |
| 45 mg, N=14 | 13 (93) | 44.5 (23.4, 45.0) | 11 (79) | 43.5 (16.6, 45.0) |
| 60 mg, N=14 | 8 (57) | 42.6 (14.2, 59.3) | 5 (36) | 56.5 (14.7, 59.5) |
| Starting Dose . | MCyR . | MMR . | ||
|---|---|---|---|---|
| N (%) | Dose intensity*, Median (min, max) | N (%) | Dose intensity*, Median (min, max) | |
| 4 mg, N=3 | 2 (67) | 3.7 (3.5, 3.9) | 1 (33) | 14.3 (14.3, 14.3) |
| 15 mg, N=7 | 5 (71) | 14.8 (14.7, 23.7) | 4 (57) | 15.0 (14.7, 36.6) |
| 30 mg, N=5 | 3 (60) | 27.4 (10.6, 29.6) | 1 (20) | 29.9 (29.9, 29.9) |
| ≤30 mg, N=15 | 10 (67) | 14.8 (3.5, 29.6) | 6 (40) | 15.0 (14.3, 36.6) |
| 45 mg, N=14 | 13 (93) | 44.5 (23.4, 45.0) | 11 (79) | 43.5 (16.6, 45.0) |
| 60 mg, N=14 | 8 (57) | 42.6 (14.2, 59.3) | 5 (36) | 56.5 (14.7, 59.5) |
*Dose intensity (mg/day) until time of response for responders only
Talpaz:ARIAD Pharmaceuticals, Inc., BMS, Sanofi, Incyte, Pfizer: Research Funding. Cortes:ARIAD Pharmaceuticals, Inc., BMS, Novartis, Pfizer, Teva: Consultancy, Research Funding. Kantarjian:ARIAD, Pfizer, Amgen: Research Funding. Shah:ARIAD, BMS: Research Funding. Flinn:ARIAD Pharmaceuticals, Inc.: Research Funding. Hu:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Rivera:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Clackson:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Turner:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Haluska:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Druker:BMS: Research Funding; ARIAD Pharmaceuticals, Inc.: PI and co-investigator on clinical trials, PI and co-investigator on clinical trials Other; MolecularMD: Consultancy, Equity Ownership. Deininger:BMS, Novartis, Celgene, Genzyme, Gilead: Research Funding; BMS, ARIAD, Novartis, Incyte, Pfizer: Advisory Board, Advisory Board Other; BMS, ARIAD, Novartis, Incyte, Pfizer: Consultancy. Mauro:ARIAD Pharmaceuticals, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.