Abstract
Introduction:
Total (-7) or partial (7q-) monosomy 7 is the second frequent abnormality in MDS, occurring in around 12% of MDS/AML and up to 40% of therapy-associated MDS/AML. The present study was designed to analyze clinical features, prognosis and response to different therapeutic strategies in patients with -7 or del(7q) in a multicentric, retrospective cohort study.
Patients and Methods:
471 patients with MDS/AML following MDS and monosomy 7 were registered and retrospectively analyzed. The median observation time was 3.6 years. Inclusion criteria were defined as follows: Morphologic diagnosis of MDS/AML following MDS, bone marrow blast count <=30% and presence of -7 or 7q- proved by chromosome banding analysis (CBA) or fluorescence in situ-hybridization (FISH). The data was coalesced from 8 centers in London (n=140; 29.7%), Duesseldorf, (n=120; 25.5%%), Goettingen (n=118; 25.1%), Cologne (n=38; 8.1%), Freiburg (n=29; 6.2%), Munich (n=13; 2.8%), Dresden (n=10; 2.1%) and Mannheim (n=3; 0.6%). The median age in the study cohort was 66 years, 63% of patients were males. MDS/AML was therapy-associated in 53 (11%). According to IPSS-R, 9 (1.9%) were assigned to the low risk group, 39 (8.3%) to the intermediate group, 81 (17.2%) to the high-risk group and 133 (28.2%) to the very high risk group. The treatment was classified as follows: Best supportive care (BSC), low-dose Chemotherapy (LDC), high-dose chemotherapy (HDC), hypomethylating agents (HMA; either 5-azacytidine or decitabine), and others (e.g. valproic acid, steroids, lenalidomide or thalidomide). Survival analyses were performed regarding overall- (OS) as well as AML-free survival (AFS) using the Kaplan-Meier method.
Results:
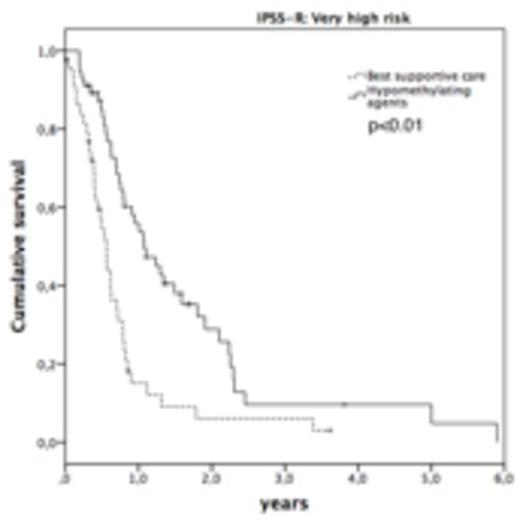
147 patients (31.2%) showed 7q-, 313 (66.5%) -7 and 11 (2.3) patients showed both abnormalities at the first cytogenetic examination. The abnormality was detected by CBA±FISH in 440 (93.4%) and by FISH only in 31 (6.6%). In the latter cases, the CBA was either unsuccessful or showed a normal karyotype. In 182 (38.6%) patients, -7/del7q was detected as a single abnormality, 77 (16.3%) showed two abnormalities and 184 (39.1%) showed a complex karyotype involving -7/7q-. As previously described (Schanz et al., 2012), untreated patients with an isolated 7q- as compared to an isolated -7 show a better prognosis regarding OS (median: 4.0 vs. 0.7 years; p<0.01) as well as AFS (median not reached vs. 2.3 years; p=0.062). Median hemoglobin level in the study cohort was 9.3 g/dl, ANC 0.98*103/μl, platelet count 73*103/μl and the median number of bone marrow blasts was 8%. Regarding the treatment, a best supportive care regimen was chosen in 195 (41%) patients. The remaining 276 (58.6) patients received 1-5 sequential therapies (one therapy: 31.6%; more than 1 therapy: 27.0%). 81 patients received an allogeneic bone marrow transplantation (ATX). Within the group of patients treated with HMA at any time of their disease (n=167), 147 (31.2%) received 5-Azacytidine, 8 (1.7%) Decitabine and 12 (2.5%) patients were treated with both drugs. As the first line therapy, 122 patients (25.9%) received HMA, 50 (10.6%) HDC, 28 (5.9%) ATX, 28 (5.9%) 11 (2.3%) LDC, and 28 (5.9%) were treated with other therapies. Patients eligible for ATX showed a significantly better prognosis as compared to any other therapy strategy: The median OS in was 2.1 years as compared to 1.1 years in non-transplanted patients (p<0.01). In patients not eligible for ATX, treatment with HMA at any course of their disease as compared to a BSC strategy was associated with a better OS (1.4 vs. 0.8 years, p=0.014). By comparing HMA to any other therapy, the OS did not differ significantly (1.4 years in HMA vs. 1.1 years in any other, p n.s.). In patients classified as very high risk according to IPSS-R, the median OS was significantly prolonged in patients receiving HMA as compared to BSC (1.1 vs. 0.6 years, p<0.01). This was also observed for the risk of AML-transformation in this subgroup of patients: The median time to AML was 1.8 years in HMA-treated patients versus 0.6 years in BSC (p=0.012).
Conclusions:
To our knowledge, the study describes the largest patient cohort with MDS/AML and monosomy 7 published to date. Further data regarding the clinical characteristics of this subgroup of patients and the treatment regimes applied will be presented in detail.
The study was supported by research funding from Celgene
Schanz:Celgene: Honoraria, Research Funding, Travel grants: Celgene, Novartis, Lilly Other. Götze:Celgene Corp, Novartis Pharma: Honoraria. Nolte:Celgene, Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Travel grants Other.
Author notes
Asterisk with author names denotes non-ASH members.