Abstract
Introduction
Extensive application of haploidentical SCT (haplo-SCT) is limited by high rate of late transplant mortality and relapse incidence associated with the delayed immune-reconstitution (IR) secondary to the procedures for severe graft-versus-host-disease (GvHD) prevention and treatment. In the past 20 years we deeply investigate the application of the paradigmatic herpes simplex virus thymidine kinase (TK) suicide gene strategy to allow the selective elimination of genetically modified donor T cells during GvHD, while sparing IR with effective graft-versus-leukemia and graft-versus-infectious effects.
Aim of the study
Here we report the incidence, characterization, stratification, treatment and outcome for both acute (a-) and chronic (c-) GvHD in haplo-SCT after TK-cells infusion.
Methods
We included for analysis 57 adult patients (pts, median age 53 years – r 17-66) who underwent an haplo-SCT according to TK-trial (Ciceri, Bonini et al, Lancet Oncol 2009; Phase III TK008, NCT0091462), between 2002 and 2014 at our Center. Data were collected from our Institutional database. A written consent was given by pts allowing the use of medical records for research in accordance with the Declaration of Helsinki.
All consecutive pts receiving graft after selection of peripheral CD34+ cells - CliniMacs one-step procedure - were selected. No immune-suppression was introduced after SCT as GvHD prophylaxis. In vivo T-cell depletion with ATG (Fresenius) was administered in all pts.
Donor lymphocytes genetically engineered to express the TK gene were infused in 34/57 pts (median 2 infusion/pts), 25/34 achieved IR (median time from SCT 84 days – r 18/182; median time from last TK-cells infusion 27 days – r 13/42).
Results
Twelve of 25 immune-reconstituted pts developed a-GvHD (grade I–IV; median time of onset 84 days post SCT – r 20/162; 19 days post last TK-infusion – r 8/54) and one developed c-GVHD. Direct association of TK-cells and GvHD was confirmed by vector-encoded protein immunostaining of lymphocytes infiltrating affected lesions.
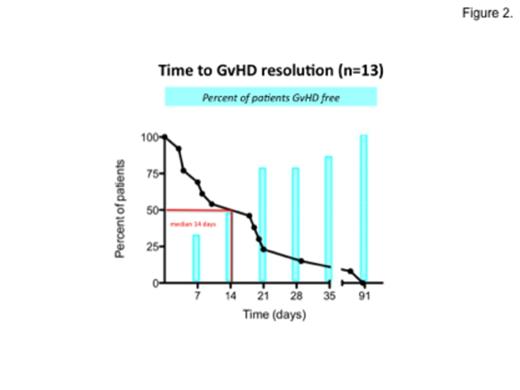
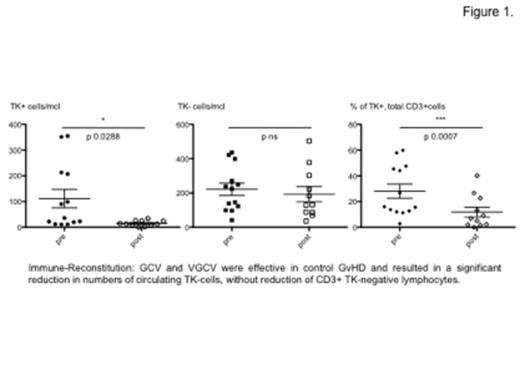
Eleven pts needed GvHD treatment: 4 pts received ganciclovir iv (GCV 5 mg/Kg/12h/14 days), 7 pts valganciclovir per os (VGCV 900 mg/12h/14 days). Both GCV and VGCV were effective in control clinical manifestations of GvHD in a median of 14 days (see figures 1. and 2.) and resulted in a significant reduction in numbers of circulating TK-cells, without reduction of CD3+ TK-negative lymphocytes maintaining long-term IR (see table). In 5 pts additional concomitant treatment with low-dose steroid (prednisone <0.5mg/kg per day for a median of 2 weeks) was given.
A pt who presented severe gut and liver GvHD and one who received at SCT an high dose of unmanipulated lymphocytes (5.4x105/Kg) – were successfully treated with a combined therapy of prednisone and cyclosporine or rapamicine in association with GCV.
Patient TK44 developed a severe classic de novo c-GVHD, with sclerodermatous lichenoid skin and mouth features plus moderate dry-eye symptoms that was successfully treated with VGCV and a transient course of mycophenolate mofetil (2 g per day) over a 2 months period.
No cases of quiescent or progressive c-GvHD was observed after a median follow-up of 679 days (r 139/4035).
Conclusion
In our 12-years experience we can confirm that infusion of TK-cells is effective in accelerating IR while controlling GvHD, providing a long-term immunosuppressive therapy free survival in absence of GvHD related deaths or long-term complications.
Despite a consistent reduction in TK-cell numbers, the GCV/VGCV treatment of GVHD did not impair or prevent long-term IR.
| TK-cells infused / Kg x107 | Time from SCT to GvHD (days) | Time from last TK-cells to GvHD (days) | Description (acute – chronic, grade) | Before GCV/VGCV | After GCV/VGCV | GvHD outcome ,days after 1st dose of GCV/VGCV | |||||
| TK+ cells/mcl | TK-cells/mcl | % of TK+, tot CD3+cells | TK+ cells/mcl | TK-cells/mcl | % of TK+, tot CD3+cells | ||||||
| TK5 | 1 | 91 | 15 | A II | 36 | 248 | 12.7 | 7 | 378 | 1.8 | CR 21 |
| TK6 | 1 | 98 | 51 | A I | 213 | 270 | 44.1 | / | / | / | CR NA |
| TK8 | 10 | 20 | 17 | A IV | 207 | 224 | 57.9 | 24 | 36 | 40.2 | CR 20 |
| TK16 | 2.2 | 20 | 8 | A II | 90 | 99 | 47.6 | 25 | 69 | 26.6 | CR 4 |
| TK20 | 2.4 | 30 | 28 | A II | 22 | 139 | 13.7 | 13 | 129 | 9.2 | CR 29 |
| TK25 | 0.4 | 162 | 14 | A II | 23 | 123 | 16 | 13 | 184 | 6.8 | CR 7 |
| TK38 | 1 | 110 | 54 | A III | 99 | 436 | 15.6 | 15 | 503 | 2.0 | CR 10 |
| TK44 | 1 | 159 | 146 | C severe | 12 | 399 | 2.9 | 0 | 303 | 0 | CR 84 |
| TK47 | 1 | 90 | 19 | A II | 351 | 422 | 45.4 | 34 | 222 | 13.3 | CR 3 |
| TK50 | 1 | 66 | 41 | A II | 355 | 238 | 59.9 | 26 | 88 | 22.8 | CR 18 |
| TK1007A | 1.2 | 117 | 30 | A I | 20 | 146 | 12 | / | / | / | CR NA |
| TK1011A | 1.06 | 78 | 20 | A II | 11 | 97 | 11.3 | 7 | 131 | 5 | CR 90 |
| TK1014A | 0.96 | 15 | 10 | A II | 11 | 41 | 26.8 | 2 | 85 | 2.3 | CR 8 |
| TK-cells infused / Kg x107 | Time from SCT to GvHD (days) | Time from last TK-cells to GvHD (days) | Description (acute – chronic, grade) | Before GCV/VGCV | After GCV/VGCV | GvHD outcome ,days after 1st dose of GCV/VGCV | |||||
| TK+ cells/mcl | TK-cells/mcl | % of TK+, tot CD3+cells | TK+ cells/mcl | TK-cells/mcl | % of TK+, tot CD3+cells | ||||||
| TK5 | 1 | 91 | 15 | A II | 36 | 248 | 12.7 | 7 | 378 | 1.8 | CR 21 |
| TK6 | 1 | 98 | 51 | A I | 213 | 270 | 44.1 | / | / | / | CR NA |
| TK8 | 10 | 20 | 17 | A IV | 207 | 224 | 57.9 | 24 | 36 | 40.2 | CR 20 |
| TK16 | 2.2 | 20 | 8 | A II | 90 | 99 | 47.6 | 25 | 69 | 26.6 | CR 4 |
| TK20 | 2.4 | 30 | 28 | A II | 22 | 139 | 13.7 | 13 | 129 | 9.2 | CR 29 |
| TK25 | 0.4 | 162 | 14 | A II | 23 | 123 | 16 | 13 | 184 | 6.8 | CR 7 |
| TK38 | 1 | 110 | 54 | A III | 99 | 436 | 15.6 | 15 | 503 | 2.0 | CR 10 |
| TK44 | 1 | 159 | 146 | C severe | 12 | 399 | 2.9 | 0 | 303 | 0 | CR 84 |
| TK47 | 1 | 90 | 19 | A II | 351 | 422 | 45.4 | 34 | 222 | 13.3 | CR 3 |
| TK50 | 1 | 66 | 41 | A II | 355 | 238 | 59.9 | 26 | 88 | 22.8 | CR 18 |
| TK1007A | 1.2 | 117 | 30 | A I | 20 | 146 | 12 | / | / | / | CR NA |
| TK1011A | 1.06 | 78 | 20 | A II | 11 | 97 | 11.3 | 7 | 131 | 5 | CR 90 |
| TK1014A | 0.96 | 15 | 10 | A II | 11 | 41 | 26.8 | 2 | 85 | 2.3 | CR 8 |
Bonini:MolMed S.p.A.: Consultancy. Colombi:MolMed: Employment. Lambiase:MolMed S.p.A: Employment. Bordignon:MolMed: Employment.
Author notes
Asterisk with author names denotes non-ASH members.