Abstract
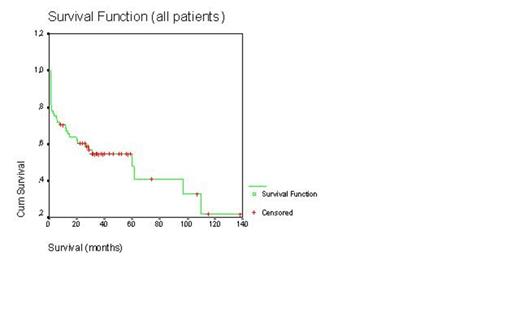
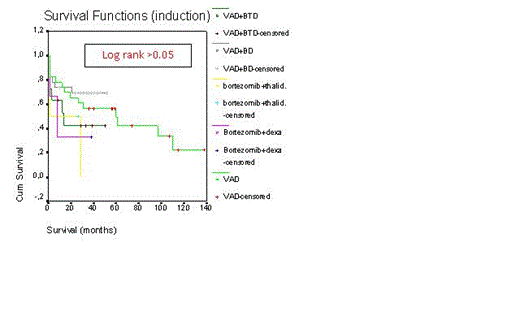
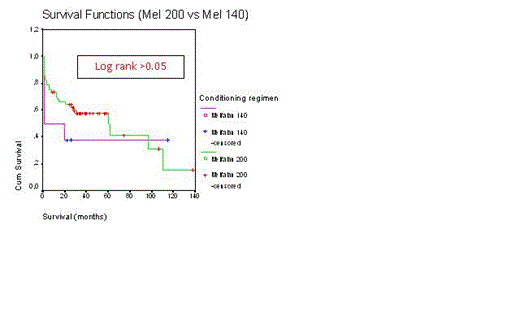
Even today autologous stem cell transplantation with high-dose melphalan is the most effective treatment for malignant plasma cell disorders such as multiple myeloma (MM) and primary amyloidosis patients less than 65-70 years old. In last 10 years, combination therapy with dexamethasone (D), bortezomib (B), and immunomodulatory drugs such as thalidomide (T) and lenalidomide provided more complete remission rate and overall survival duration. We retrospectively evaluated our transplant center’s results of peripheral blood stem cell transplantation (PBSCT) in the treatment of malignant plasma cell disorders. PBSCT with high-dose melphalan was performed to 62 patients with MM and 2 patients with primary amyloidosis. 40 were male patients 24 female patients. The mean age of patients and age ranges were 59±10 and 40-80 years, respectively. All PBSCT were autologous except one syngeneic. In our country, bortezomib, thalidomide, or lenalidomide can not be applied as first line treatment in transplant eligible patients, unless patients have 13q or 17p deletion or progression occurs after the use of 2 cycles of alkylating agents. So 23 patients with VAD, 23 patients with BD after 2 cycles VAD, 11 patients with BTD after after 2 cycles VAD, 4 patients with BTD, and 3 patients were treated with BD as induction therapy. Melphalan-200 mg/m2 and melphalan-140 mg/m2 as conditioning regimen were applied to 56 and 8 patients, respectively. Only 6% of PBSCT were performed in relapse/refractory patients. Mean time from diagnosis to transplantation was 10±15 months (range 2.5-90 months). Transplant-related mortality rate was 14%. Relaps rate was 33%. Relapse/progresyon-related mortality was 14%. Median time from transplantation to relapse was 25±8 months (range 3-106 months). There were no differences for median relapse duration between induction therapies and conditioning regimens (Log rank >0.05, for both). Median overall survival for all patients was 60±18 months (Figure-1). Median survival duration was not different between induction therapies (Log rank p>0.05, Figure-2). Median overall survivals were 60±24 months for VAD, 38±5 months for BD after VAD, 14±8 months for BTD after VAD, 16±9 months for BD, and 15±9 months forBTD, respectively. Moreover there was no difference for median overall survival between mel-200 and mel-140 (Log rank p>0.05, Figure-3). Median overall survivals were 60±18 months for mel-200 and 46±19 months for mel-140, respectively. At 60 months follow-up, 50% of patients were alive. In our center, PBSCT with high-dose melphalan was effective treatment with 50% rate of 5-year overall survival in malignant plasma cell disorders.
Overall survival (Conditioning regimens-Mel-200 vs Mel-140)
Log rank >0.05
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.