Abstract
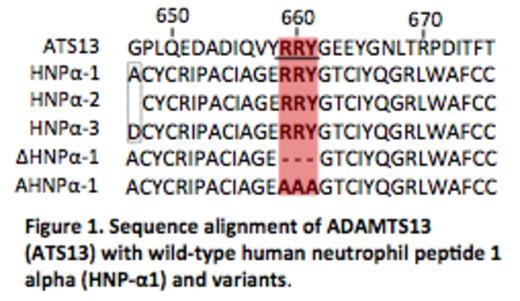
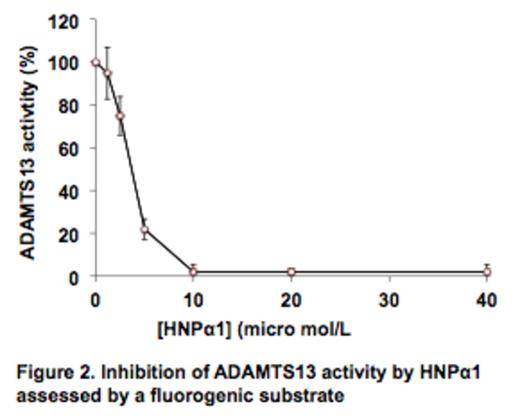
Thrombotic thrombocytopenic purpura (TTP) is a potentially fatal syndrome associated with severe deficiency of plasma ADAMTS13 activity resulting from either mutations or autoantibodies. However, patients with severe ADAMTS13 deficiency do not always develop TTP. Rather, a trigger, such as infection or inflammation, often precedes the onset of the TTP syndrome. We hypothesized that antimicrobial human neutrophil peptides 1-3 (HNPα1-3) or α-defensins, the most abundant proteins in the granules of neutrophils, which are released at site of inflammation, activate platelets, and inhibit fibrinolysis, might help to initiate TTP. This question arose because we noted that the amino acid sequences of HNPα1-3, which are nearly identical except for one residue at the N-terminus, all contain a motif (RRY) similar to exosite 3 (659RRYGEEY665) in the spacer domain of ADAMTS13 (Fig. 1) that was shown to be critical for recognition of von Willebrand factor (VWF). Here, we found that both purified and synthetic HNPα1 bind to FRETS-VWF73 and plasma-derived VWF and inhibit proteolytic cleavage of these substrates in a concentration-dependent manner. At the final concentrations of 10 micro mol/L and 150 micro mol/L, HNPα1 completely abolished the cleavage of FRETS-VWF73 (IC50=3.5 micro mol/L) (Fig. 2) and VWF (IC50=75 micro mol/L) (not shown), respectively. Such concentrations are readily attained locally after systemic infection. Deletion or alanine substitution within the RRY motif of HNPα1 completely abolished its ability to inhibit ADAMTS13 activity assessed by FRETS-VWF73 and VWF multimer analysis. This suggests that an interaction of the RRY motif in HNPα1 with the central A2 domain of VWF is required to mediate its inhibition. In support of this hypothesis, HNPα1 interacts with a human monoclonal antibody against ADAMTS13 scFv (the single chain fragment of variable region) designated 4-20, but not scFv3-1, both isolated by phage display from patients with acquired autoimmune TTP. Hydrogen-deuterium exchange mass spectrometry has shown that the binding site for scFv4-20, but not scFv3-1, contains the RRY sequence. These results suggest that HNPα1-3 released from neutrophils following infection or inflammation may inhibit residual plasma ADAMTS13 activity in vivo similar to anti-ADAMTS13 autoantibodies by interfering with its interaction with VWF, thereby triggering the onset of hereditary and acquired autoimmune TTP. Our findings suggest a potential novel link between systemic inflammation and the pathogenesis of TTP and possibility other thrombotic sequelae.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.