Abstract
RSV is a common infection in infancy and childhood. Mortality from RSV is reported to be < 0.5% in healthy children in comparison to 10-12.5% in children following HSCT (1. Chavez-Bueno S et al. Pediatr Infect Dis J 2007 26:1089-1093). There is currently no standard for management of RSV in pediatric patients with hematologic malignancies/HSCT. Many institutions have their own protocols for managing these patients. Symptomatic management is common and the use of antiviral (AV)-ribavirin, Immunomodulators like palivizumab (PVZ) and IVIG is widely debated & inconsistent in practice.
Ribavirin is a guanosine analogue with AV activity. It may be administered orally (PO), intravenously (IV) or aerosolized (AR). AR is approved by Food and Drug Administration (FDA) for treatment of RSV in high-risk (HR) infants and children. In the adult HSCT setting, retrospective data has shown the benefit of AR for RSV, for both decreasing the likelihood of progression from upper respiratory infection (URTI) to lower respiratory tract infection (LRTI) and reduction in RSV-related mortality (Shah JN et al. Blood 2011; 117:2755-2763). However in the pediatric HSCT setting the benefit of AR is less clear. AR is expensive and a known teratogenic. Healthcare workers, parents, others caring for the patient stand to be exposed to Ribavirin. The delivery mechanisms to the lower airways are inefficient. AR tends to clog the endotracheal tube and cannot be used with high frequency oscillators. Ribavirin has side effects such as sudden deterioration of respiratory response. AR therapy poses many challenges and is not always feasible.
PVZ is a 95% humanized and 5% murine monoclonal antibody that provides both neutralizing and fusion-inhibitory activity against RSV. It binds to the linear epitope of the Fusion glycoprotein in the A antigen site. PVZ is FDA approved for the prevention of RSV related LRTI in HR pediatric patients. In the HSCT setting, PVZ has been utilized off-label both for prophylaxis and treatment of RSV. PVZ +/- AR has been shown to be effective in decreasing morality among HR children including those requiring HSCT (1). The treatment of pediatric patients requiring HSCT has been derived from adult protocols due to a lack of randomized clinical trials in children. A recent retrospective analysis showed a reduction in mortality and progression to LRTI in 59 pediatric cancer patients with RSV when PVZ was added to the treatment regimen. (Chemaly RF et al. J Pediatr Hematol Oncol 2014; 36 (6): e376-81).
To evaluate the current practice, our institution surveyed several hospitals to determine institutional standards of care for treating HSCT patients with RSV (both adults and children). Responses are shown in Table 1.
| Hospital, patient population (Adult/Pediatric/Both) . | AR . | PO Ribavirin . | PVZ Treatment . | PVZ prophylaxis . |
|---|---|---|---|---|
| 1, A | Y - Inpatients | Y - Outpatients | N | N |
| 2,B | Y (Pre-engraftment, severe LRTI) | Y (Post-engraftment, URTI, mild LRTI) | N | ? |
| 3, A | Y | N | N | N |
| 4, P | Y – 5 (URTI) vs. 7days (LRTI) | N | Y (LRTI/URTI High risk) | Y in <2yr |
| 5, A | N | Y | N | NA |
| 6, A | Y (Critical) | Y | N | NA |
| 7, A | N | Y [Moderate, severe immune deficiency (SID)] | N | NA |
| 8, P | Y (HR + URTI/LRTI) | N | Y (LRTI w high risk) | Y <2yr |
| 9, A | Y | Y (URTI w No HR) | N | N |
| 10, B | Y | N | N | N |
| Hospital, patient population (Adult/Pediatric/Both) . | AR . | PO Ribavirin . | PVZ Treatment . | PVZ prophylaxis . |
|---|---|---|---|---|
| 1, A | Y - Inpatients | Y - Outpatients | N | N |
| 2,B | Y (Pre-engraftment, severe LRTI) | Y (Post-engraftment, URTI, mild LRTI) | N | ? |
| 3, A | Y | N | N | N |
| 4, P | Y – 5 (URTI) vs. 7days (LRTI) | N | Y (LRTI/URTI High risk) | Y in <2yr |
| 5, A | N | Y | N | NA |
| 6, A | Y (Critical) | Y | N | NA |
| 7, A | N | Y [Moderate, severe immune deficiency (SID)] | N | NA |
| 8, P | Y (HR + URTI/LRTI) | N | Y (LRTI w high risk) | Y <2yr |
| 9, A | Y | Y (URTI w No HR) | N | N |
| 10, B | Y | N | N | N |
Y =Used, N=Not used,?=Unsure, NA=Not Applicable
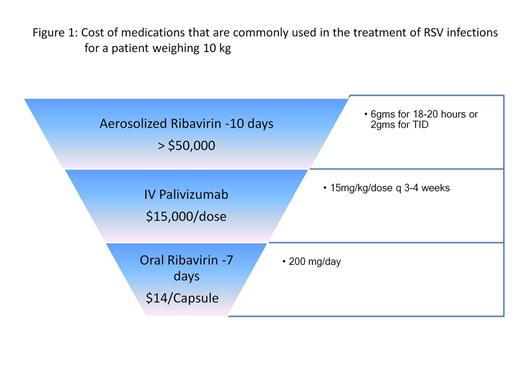
The cost of medications commonly used to treat RSV infections is shown in Figure 1.
Survey Findings:
PO ribavirin was commonly used to treat RSV URTI in responding hospitals. AR was used to treat severe LRTI. Engraftment status (pre vs. post) was used to classify severity of immunodeficiency and determine treatment. PVZ use for treatment is uncommon among the responding hospitals (2/10).
Practice at our institution
Low-risk patients with RSV (>30 days post-HSCT, minimal immunosuppression, URTI) receive only supportive care and close monitoring. Patients with RSV-LRTI or those with URTI at HR for progression (< 30 days post-HSCT, low lymphocyte counts, on immunosuppression) may be considered for AR +/- PVZ. In the last 5 years we have had 20 patients with RSV, 3 were diagnosed with RSV pre-transplant and 17 post-transplant, 1 reactivated after transplant and there was 1 death related to RSV.
Conclusion
Due to variance in practice and costs of therapies, further studies are needed to standardize treatment for children post-HSCT with RSV to reduce morbidity and mortality. In addition a stratification algorithm to better characterize those most likely to benefit from current treatment should be considered.
Off Label Use: Multi institutional survey regarding the use of Palivizumab and Ribavirin in the treatment of RSV..
Author notes
Asterisk with author names denotes non-ASH members.