Abstract
Background:
Acute myeloid leukemia (AML) is a diagnosis encompassing a diverse group of myeloid malignancies. Heterogeneous genetic etiology, together with the potential for oligoclonality within the individual patient, has made the identification of a single high sensitivity marker of disease burden challenging. PCR based assessment of WT1 expression has been extensively tested as a marker of submicroscopic AML disease burden but is not universally overexpressed in all cases. Our hypothesis was that the addition of other individually suboptimal gene expression assays to the backbone of WT1 PCR based testing in a multi-gene array format would improve prediction of relapse and survival when tested prior to allogeneic stem cell transplantation (allo-SCT).
Patients and Methods:
We identified pre-transplantation peripheral blood samples from 74 AML patients who had received allo-SCT as part of NHLBI clinical protocols performed at the NIH clinical center in Bethesda, Maryland between 1994 and 2012. All had morphological evaluation of disease status within the two months prior to transplantation and at least twelve months of documented post-SCT clinical outcomes data or until date of death if this occurred before twelve months. Transplantswere predominately myeloablative, T cell depleted, peripheral blood stem cell sibling matched allogeneic transplants with cyclosporine-based graft versus host disease prophylaxis. Peripheral blood samples from fifty healthy adults donors were used as baseline controls. All clinical protocols and permission to use blood for research were conducted in accordance with Declaration of Helsinki principles and were approved by the Institutional Review Board with written informed consent obtained from all subjects.
One microgram of total RNA isolated from cryostored peripheral blood samples was reverse-transcribed into cDNA and used for quantitative real time PCR (qRT-PCR) reactions using custom multi-gene PCR array plates (MG-MRD) and SYBR green, with normalization to c-abl. Arrays included primers for detection of WT1, CCNA1, PRTN3, PRAME, MSLN and ABL transcripts. Thresholds for positivity were established based on expression levels seen in healthy normal donors. Statistical analysis was performed using GraphPad Prism (La Jolla, CA, USA). Comparison between survival or relapse curves was performed using the Log-rank (Mantel-Cox) test.
Results:
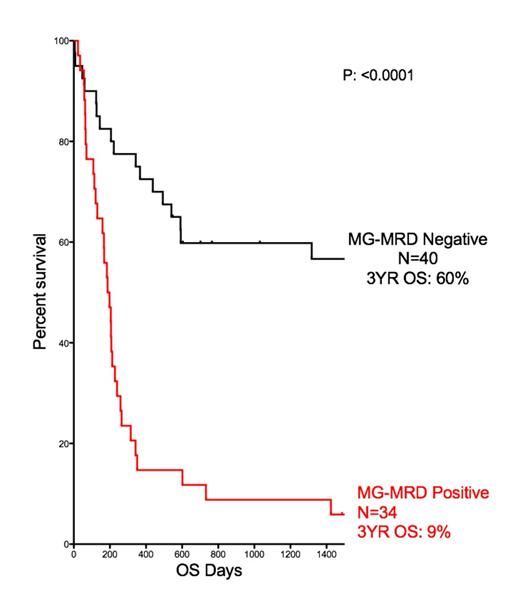
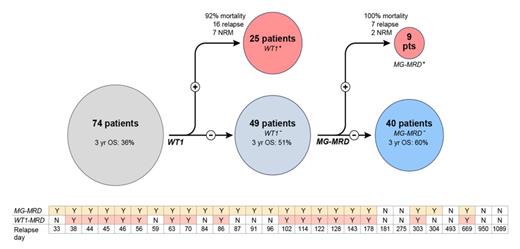
In this cohort of 74 patients the overall survival observed at three years after transplant (3yr OS) was calculated to be 36%. Traditional response criteria from examination of bone marrow risk stratified these 74 patients into two groups, the morphological CR group (n=48) with a 3yr OS of 48% and a group of 26 patents with active (refractory/refractory) disease at the time of allo-SCT with a 3yr OS of only 15%. Peripheral blood testing for WT1 overexpression identified 25 patients as positive (3 of whom had no clinically evident disease by morphology on bone marrow examination, ie; minimal residual disease, MRD). Testing using the MG-MRD assay identified an additional 9 patients from the WT1 negative group as also having residual disease (2 had morphologically evident disease, 7 with MRD). The mortality in this group of patients following transplantation was 100%. The 34 (of the total 74) patients with positive MG-MRD prior to transplantation had a three year overall survival of just 9%.
This MG-MRD positive group also experienced 22 out of a total of 24 relapses observed in the first year following transplantation. Pre-SCT MG-MRD testing correctly predicted all cases of early relapse (ie: within 100 days after allo-SCT) compared to just 57% (8/14) using WT1 alone. For the 37 patients who entered transplant in a pathological CR, and who did not suffer non-relapse mortality in the first year, the 1yr relapse rate was 24%. Pre-SCT WT1 testing had 100% positive predictive value (PPV) but only 33% sensitivity in identifying relapses in this subgroup, compared to 100% PPV and 89% sensitivity for the MG-MRD array.
Conclusion:
PCR based pre-SCT MRD testing using a multi-gene panel outperformed WT1 alone, correctly identifying 36% more patients (n=9, all of whom died) as belonging to a group at high-risk for post-SCT relapse and death. Collaborations are now being sought to validate these findings in additional cohorts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.