Abstract
Background: Patients with high-risk FL intensified with HDT/ASCT may achieve prolonged remissions. The best timing for the procedure remains controversial. Patients who are transplanted in first response show a major Progression Free Survival (PFS) and Event Free Survival (EFS) advantage compared to those treated with conventional chemotherapy. Nevertheless, no randomized studies have yet shown an overall survival (OS) benefit. Populational-based and very long-term retrospective analysis are indispensable tools to assess the actual impact on outcome of therapeutic interventions in FL. With this assumption we performed a retrospective analysis in FL patients undergoing HDT/ASCT intensification included in the GELTAMO Spanish Group Registry.
Objectives: The overall outcome as well as the clinical evolution according to the disease status at transplant, to the Follicular Lymphoma International Prognostic Index (FLIPI and FLIPI II) and to the previous exposure to Rituximab.
Series characteristics: Six hundred and sixty six patients with FL (mean age 47 years, male 49%) undergoing HDT/ASCT between 1989 and 2007 were reported to the GELTAMO registry. Patients with histological transformation at the time of HDT/ASCT, those undergoing a 2nd transplant and those with a follow-up of less than 7 years were excluded. Thus, 640 patients were included in the analysis. Median follow-up was 12.2 years from HDT/ASCT and 14.2 years from diagnosis. Follow-up from HDT/ASCT was over 16 years for 153 patients (3rd quartile). The median time from diagnosis to HDT/ASCT was 1.8 years. Two hundred and forty-seven patients (38%) never achieved a complete remission (CR) before HDT/ASCT. Two hundred patients (31%) received HDT/ASCT after achievement of first CR (CR1), 43% of them requiring more than one chemotherapy line to achieve CR1; 26% in 2nd CR, 5% in 3rd CR, 21% in 1st partial response (PR), 12% in chemosensitive recurrence, and 5% with active disease. Of the 321 patients assessable for the FLIPI, 33% had a low-risk (LR), 36% an intermediate-risk (IR), and 45% a high-risk (HR) score; and of the 305 patients assessable for the FLIPI II, 22% had a LR, 38% an IR and 40% a HR. Of the 127 patients in CR1 assessable for the FLIPI, 28% had a LR, 40% an IR, and 32% a HR; of the 115 patient assessable for FLIPI II, 14% had a LR, 46% an IR, and 40 % a HR. One third of patients received Rituximab prior to transplant.
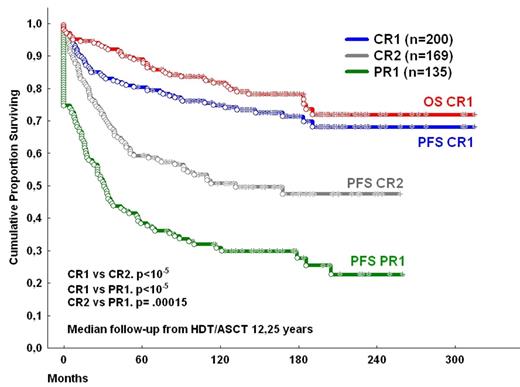
Results: Median PFS and OS were 9.4 and 21.3 years, respectively. Patients transplanted in CR1 achieved significantly better final PFS (68%) and OS (73%), than those transplanted in 2nd CR (median PFS 110 months (mo.) and final OS 58%; P <.0005) and the latter ones better than those transplanted in 1st PR (PFS median PFS 31 mo. and median OS 118 mo.; P < .0005) (figure 1). Neither FLIPI1 nor FLIPI2 reached statistical significance in patients transplanted in CR1 (P= .5 and P= .2 for PFS and OS, FLIPI 1 comparisons; P= .47 and P= .1 for PFS and OS, FLIPI 2 comparisons, respectively). Although in the global series patients who received Rituximab prior to HDT/ASCT had a better prognosis than those who did not (median PFS not reached vs median PFS 92 mo, respectively, P= .0005; median OS not reached vs median PFS 246 mo, respectively, P= .0012), treatment with Rituximab has no prognostic impact in cases transplanted in CR1. Only 6 patients died beyond 10 years of follow-up, (1 disease progression, 3 second malignancies, 2 unrelated causes). The accumulated incidence of second malignancies of the global series was 12%. A plateau was observed in the PFS and OS curves for patients transplanted in CR1 beyond 15.9 years from transplantation (figure 1).
Conclusions: To the best of our knowledge there is no study on the therapeutic impact in the evolution of LF offering such a long follow-up. HDT/ASCT is a good option of consolidation for those patients who achieve a good quality of response with chemotherapy. The finding of a plateau beyond 15.9 years for patients transplanted in first remission suggests that a significant number of patients from this group may never relapse and could be cured, even those with poor initial features.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.