Abstract
Given the excellent survival rates for early-stage Hodgkin lymphoma (HL), the young age of many patients, and concerns regarding acute and late treatment-related toxicities, there is a desire to have a predictive tool that enables therapy to be tailored toward the individual patient. Early (or interim) 18F-fluorodeoxyglucose positron emission tomography with computerized tomography (FDG-PET/CT), as a test of tumor sensitivity to ongoing/planned therapy, has been shown to be prognostic for survival in HL. Based on results of interim FDG-PET/CT, therapy may be subsequently modified through minimization or via intensification for low- and high-risk patient populations, respectively (ie, response-adapted therapy). Important data have been generated to standardize the interpretability and reproducibility of interim FDG-PET/CT (eg, the Deauville 5-point system), and observational and noncontrolled prospective studies have produced evidence supporting the hypothesis that response-adapted therapy may potentially serve as a predictive tool. Furthermore, results from noninferiority phase 3 clinical trials randomizing early-stage HL patients with negative interim FDG-PET/CT to combined modality therapy versus chemotherapy alone have been reported. The current collective findings from these randomized early-stage HL studies have shown that acute relapse rates are lower with combined modality therapy, even in patients with negative interim FDG-PET/CT. Additional randomized response-adapted studies are ongoing and novel FDG-PET/CT applications involving quantitative techniques and innovative imaging modalities are being investigated to identify more robust imaging biomarkers. Treatment of early-stage HL remains a clinical management choice for physicians and patients to make with consideration of acute and long-term outcomes.
Introduction
The large majority of patients with early-stage Hodgkin lymphoma (HL) will achieve complete remission (CR) and be cured with current treatment paradigms.1 A common treatment strategy for early-stage HL involves combined modality therapy (CMT) sequencing consolidative radiotherapy after induction chemotherapy, although chemotherapy alone is a viable option. The number of chemotherapy cycles (ie, 2-6) and radiation dose (ie, 20-30 grays [Gy]) are dependent in part on clinical risk factors.2 Despite disease-specific survival rates of 90%–95% for early-stage HL with current treatment paradigms, treatment-related toxicities remain a concern. These include increased risk of late effects such as second cancers and arterial disease,3-5 as well as a negative impact on quality of life.6,7 Significant efforts have been made to decrease the amount and intensity of therapy to potentially mitigate acute and long-term toxicities.4,8 Conversely, a small subset of early-stage patients will have primary refractory disease or experience relapse and ultimately die of the disease. There is an interest to identify these high-risk groups earlier in the treatment course to potentially institute modified and/or intensified therapy, which may lead to improved outcomes. In both clinical scenarios, it is desirable to have a prognostic tool that may predict patient outcome and allow therapy to be tailored toward the individual patient.
18F-fluorodeoxyglucose positron emission tomography with computerized tomography (FDG-PET/CT) is a functional imaging modality that has become a standard tool complementing contrast enhanced CT (CECT) scans in the diagnosis and management of HL.9 Several studies have shown that FDG-PET/CT more accurately identifies the correct pretreatment stage in HL compared with CECT, although FDG-PET/CT upstages disease from early to advanced stage in only 10%–15% of patients in whom treatment is ultimately modified.10,11 After completion of the intended treatment, FDG-PET/CT is also usually able to distinguish viable tumor cells from fibrosis or necrosis in a residual mass. FDG-PET/CT may have its greatest impact, however, in the prediction of patient outcome with use of early (interim) imaging. Results from noncontrolled studies of interim FDG-PET/CT (eg, after 2 cycles of chemotherapy) as a test of tumor sensitivity of planned/ongoing therapy have been shown to be prognostic of survival.12-18
Numerous studies have been published over the past decade regarding the clinical utility of FDG-PET/CT in the management of HL.14-22 These include important data that have been produced regarding the standardization of the reproducibility and interpretability of FDG-PET/CT, which are critical for the appropriate incorporation of this modality into routine clinical practice. In addition, observational and noncontrolled prospective studies have generated hypotheses supporting the concept of early (interim) risk assessment using FDG-PET/CT imaging for prognostication and prediction of outcome. Moreover, results of randomized phase 3 clinical trials incorporating response-adapted treatment strategies based on interim FDG-PET/CT to guide treatment decisions have been completed recently in an attempt to answer the question of whether interim FDG-PET/CT is a compass for safe navigation in HL.19 There are also multiple novel FDG-PET/CT applications using quantitative techniques, and new imaging modalities are being investigated for the potential incorporation into clinical care for better management of HL patients.
FDG-PET interpretation and reproducibility
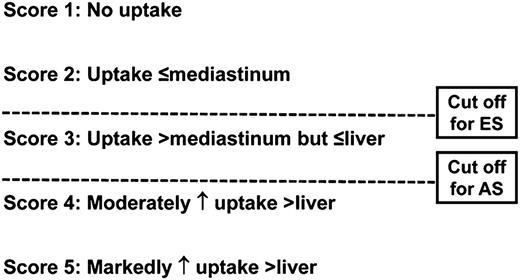
The first initiative for standardization was adopted in 2007 for the “end-of-therapy” FDG-PET/CT interpretation by the imaging subcommittee of the International Harmonization Project (IHP) in Lymphoma.23 According to these criteria, uptake greater than that seen in the mediastinal blood pool in residual masses measuring ≥2 cm was considered positive for residual lymphoma. It is important to note that these criteria were not recommended for interim FDG-PET/CT evaluation. A more accurate method for measuring response versus a dichotomous dataset would be a continuous variable with a categorical scoring system, such as the Deauville 5-point system (5PS; Figure 1).24-26
The Deauville 5PS. Shown are the criteria for interpretation of interim FDG-PET/CT. A Deauville score >3 is the most optimal cutoff for interim PET with advanced-stage HL to increase PPV if intensification of therapy is planned, whereas a cutoff <3 is desirable for nonbulky early-stage HL to enhance NPV. ES indicates early-stage; and AS, advanced stage.
The Deauville 5PS. Shown are the criteria for interpretation of interim FDG-PET/CT. A Deauville score >3 is the most optimal cutoff for interim PET with advanced-stage HL to increase PPV if intensification of therapy is planned, whereas a cutoff <3 is desirable for nonbulky early-stage HL to enhance NPV. ES indicates early-stage; and AS, advanced stage.
A scoring system that enables different cutoffs (ie, thresholds for positive vs negative) is desirable to assess chemotherapy sensitivity versus response. Such a scoring system is also adaptable as study goals and end points change. A high positive predictive value (PPV) using a higher threshold (ie, liver uptake) is preferred for therapy intensification to minimize overtreatment and toxicity and to decrease the rate of false-positives, whereas a high negative predictive value (NPV) using a lower threshold (ie, mediastinal blood pool) may be used to decrease the intensity of therapy to minimize undertreatment. To address these needs, the Deauville 5PS was developed to serve as a categorical reading scheme that has different positivity thresholds to adjust for the intended treatment end points (Figure 1).24-26 In addition, by using the patient as his/her own control with a reference organ with relatively consistent metabolic activity (eg, mediastinal blood pool and liver), it minimizes interreader subjectivity and reduces interdevice inconsistency.27
In a study by Le Roux et al, the improved prognostic value of the Deauville 5PS was confirmed.28 This study showed that the NPV was high regardless of the criteria applied, but that the use of a higher threshold for a positive interim FDG-PET/CT led to an increased PPV. The best result was obtained using Deauville 5PS criteria, which increased the PPV from 19% to 45%. Furthermore, interim FDG-PET/CT correlated strongest with progression-free survival (PFS) using 5PS criteria (P < .0001). The reproducibility of Deauville 5PS was also confirmed in an international multicenter study of a retrospective cohort of 260 advanced-stage HL patients imaged after 2 of 6 intended cycles (ie, PET-2) of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), with no treatment change based on PET-2 results.29 The sensitivity, specificity, NPV, and PPV for PET-2 were 73%, 94%, 94%, and 73%, respectively. After a mean follow-up of 27 months, the 3-year failure-free survival was 28% for PET-2+ patients and 95% for PET-2− patients (P < .0001). The binary concordance between paired reviewers was high (Cohen κ = 0.84). It should also be appreciated that the NPV and PPV of FDG-PET/CT in HL may be disease- and treatment-specific and the aforementioned results should not be automatically applied outside of HL and ABVD, respectively.
Current treatment paradigms for early-stage HL
A common current treatment recommendation for early-stage HL patients with a favorable risk profile involves CMT consisting of 2-3 cycles of ABVD followed by 20-30 Gy of involved field radiotherapy (IFRT) or involved site RT (ISRT). Commonly recommended therapy for early-stage patients with an unfavorable (intermediate) risk profile includes 4 cycles of chemotherapy followed by 30 Gy IFRT/ISRT. In addition, chemotherapy alone for 4-6 cycles (without radiation) has been shown to be a valid option for the treatment of early-stage HL.8
From 2002 to 2005, there were 4 published randomized clinical trials that compared CMT versus chemotherapy alone for the treatment of adult early-stage HL.30-33 In each of these studies, disease control (ie, freedom from disease progression, freedom from treatment failure, or event-free survival) was better with CMT versus chemotherapy with absolute improvements ranging from 3% to 7%. Overall survival (OS) rates were similar in each study, although final analysis of the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) Eastern Cooperative Oncology Group (ECOG) HD.6 study showed superior OS for chemotherapy alone at 12 years due to increased late events/toxicity in the CMT arm.34 A recent analysis that combined individual patient data from the NCIC-CTG HD.6 and German Hodgkin Study Group (GHSG) HD10/HD11 studies showed that disease control (ie, 8-year time to treatment progression) was improved by 6% with CMT versus chemotherapy alone, whereas OS rates were identical at 95%.35 Similar data regarding the improvement in disease control (ie, event-free survival) with similar OS was seen in a pediatric HL study randomizing patients who achieved CR on CT to IFRT or no radiotherapy.36
Improvement in acute disease control (eg, PFS) is well documented in early-stage HL patients who receive CMT versus chemotherapy alone; however, this has not translated to an improvement in OS. In addition, there are late adverse effects to consider such as arterial disease and second cancers that occur. Therefore, the preferred treatment of HL patients with early-stage disease continues to be strongly debated in part because of the overarching goal of long-term OS with preserved quality of life.37 New scientific advances have been sought to identify select, low-risk patients in whom radiation may be obviated and/or less chemotherapy delivered. The functional imaging modality, FDG-PET/CT, has been examined as a tool to direct when treatment should be deintensified or escalated based on interim results.
Interim PET/CT in early-stage HL
Observational and prospective studies without treatment modification
FDG-PET/CT may provide prognostic information at the individual patient level, allowing early in vivo evaluation of chemotherapy sensitivity. It should be highlighted that most initial observational studies reporting on the potential value of interim FDG-PET/CT as a response predictor included mixed profiles of HL patients with divergent risk factors.9 Furthermore, there is comparatively much less data regarding the predictive value of interim PET in early-stage HL versus advanced-stage HL, especially in favorable early-stage HL.12,14,15,18,22,38 In the observational study by Gallamini and Hutchings that ignited an intense interest into response-adapted therapy in HL, only a minority of patients had early-stage disease and most of these patients had adverse risk factors (ie, unfavorable/intermediate early-stage HL).12
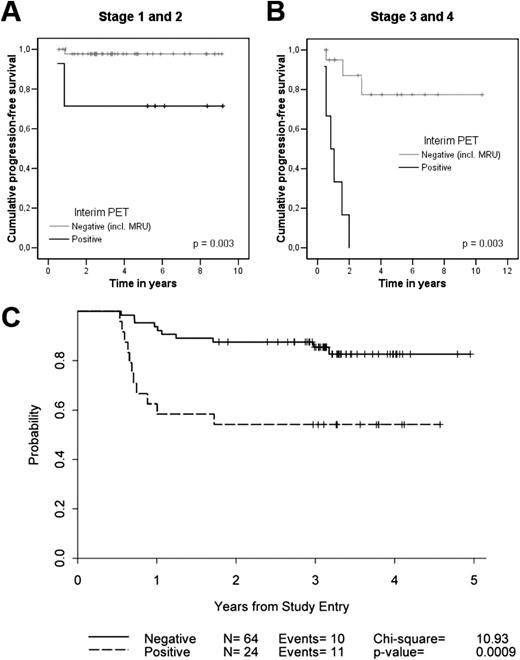
In a retrospective analysis of 85 HL patients who had interim FDG-PET/CT after 2-3 cycles of ABVD, the predictive power of FDG-PET/CT was much less robust for early-stage versus advanced-stage HL patients (Figure 2A,B).39 Interim FDG-PET/CT was prognostic for 2-year PFS among the 57 early-stage HL patients (P = .003), but only 2 of 7 interim FDG-PET/CT-positive patients with early-stage relapsed. Interestingly, Ann Arbor stage retained strong prognostic significance on multivariate analysis with interim FDG-PET/CT included as a covariate. In a subsequent prospective analysis of patients with early- and advanced-stage HL, extranodal disease and a positive interim FDG-PET/CT were found to be predictive of outcome.13 Among patients with early-stage disease, none with a negative PET-2 progressed (0/26) and only 1 of 5 with positive PET-2 experienced progression.
Prognostication of FDG-PET/CT in early-stage HL. Shown is the PFS according to the result of interim FDG-PET/CT (status-post 2-3 ABVD cycles) of 57 early-stage (A) and 28 advanced-stage (B) HL patients. Treatment was continued regardless of FDG-PET/CT result. (Reprinted with permission from Hutchings et al.39 ) (C) PFS for 88 patients with early-stage nonbulky HL treated on a US Cooperative group phase 2 study using AVG frontline therapy. Incl indicates including; MRU, minimal residual uptake. (Reprinted with permission from Straus et al.38 )
Prognostication of FDG-PET/CT in early-stage HL. Shown is the PFS according to the result of interim FDG-PET/CT (status-post 2-3 ABVD cycles) of 57 early-stage (A) and 28 advanced-stage (B) HL patients. Treatment was continued regardless of FDG-PET/CT result. (Reprinted with permission from Hutchings et al.39 ) (C) PFS for 88 patients with early-stage nonbulky HL treated on a US Cooperative group phase 2 study using AVG frontline therapy. Incl indicates including; MRU, minimal residual uptake. (Reprinted with permission from Straus et al.38 )
Other investigators have reported similar PFS differences for interim FDG-PET/CT+ and FDG-PET/CT− groups (P = .57) in nonbulky limited-stage HL patients treated with standard therapy.14 This study was limited by its retrospective design and variable FDG-PET/CT timing (intervals of PET 2-4), although the results have since been corroborated.15,18 Sher et al reported a 2-year failure-free survival of 92% versus 69% for patients undergoing consolidation radiation therapy (RT) versus no RT for residual FDG-PET/CT avidity after completion of ABVD, indicating the potential efficacy of RT to a residual mass after chemotherapy.62 It should be highlighted that the efficacy of treatment is a crucial factor that may significantly alter the predictive value of FDG-PET/CT. In a prospective study of 88 patients with early-stage nonbulky HL treated with a nonstandard chemotherapy regimen of AVG (doxorubicin, vinblastine, gemcitabine), 2-year PFS rates were 88% and 54% for FDG-PET-2− and FDG-PET-2+ groups, respectively (P = .0009) (Figure 2C).38 Although PPV was better, the NPV (86%) appeared to be inferior to previously published early-stage HL data (95%–100%) in part due to the lower CR rate achieved with the AVG regimen (81%) compared with ABVD (94%).
Collectively, initial reports of interim FDG-PET/CT for early-stage HL demonstrated a consistently high NPV and a low to moderate PPV in relation to treatment outcome. The high incidence of inflammatory processes, particularly in those with bulky disease, may contribute to a significant number of false-positive FDG-PET/CT results. Thus, the most attractive application of a PET-response–adapted strategy in early-stage HL is likely de-escalation of therapy (eg, omission of consolidative radiation therapy) for those with negative interim FDG-PET/CT, while modified and/or escalated therapy may be more challenging due to the modest PPV of positive interim FDG-PET/CT.
Phase 2 clinical trials using response-adapted strategies in early-stage HL
There have been only a handful of phase 2 clinical studies completed using a response-adapted strategy with interim FDG-PET/CT for early-stage HL. Le Roux et al reported results in patients with early- and advanced-stage HL patients undergoing treatment with a response-adapted strategy after 4 cycles of ABVD (ie, PET-4; Table 1).28 In stage I/II nonbulky patients (n = 26), PET-4− patients without progressive disease on CT or patients with CR on CT regardless of FDG-PET/CT findings received only IFRT. In patients with bulky stage I/II and advanced-stage disease (n = 44), those with negative PET-4 received 4 more cycles of ABVD. The remaining 28 patients with positive PET-4 and no CR on CT underwent autologous stem cell transplantation. The NPV and PPV with PET-4 for 2-year PFS were 95% and 16%, respectively (P < .0001). The low PPV reflects the likely negative impact that therapeutic intensification had on the predictive value of interim FDG-PET/CT results.
Dann et al reported preliminary results from an ongoing phase 2 study examining response-adapted therapy that included early-stage HL (Table 1),40 whereas other phase 2 prospective studies have contained only a small minority of early-stage patients. Two US CALGB-led early-stage response-adapted studies await long-term follow-up and completion of patient accrual (Table 1). CALGB 50801 (www.ClinicalTrials.gov identifier #NCT01118026) is an important clinical trial in that it is one of the few prospective response-adapted studies in early-stage HL that is studying patients with bulky disease.
Completed phase 3 clinical trials using response-adapted strategies in early-stage HL
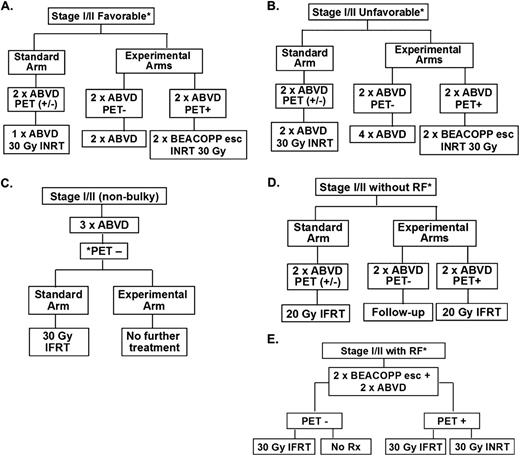
Recently completed response-adapted randomized studies are detailed and depicted in Figure 3 and Table 2. The European Organisation for Research and Treatment of Cancer (EORTC)-led H10F and H10U studies randomized patients with favorable and unfavorable early-stage HL (according to EORTC definitions) to FDG-PET-based versus non-PET-based treatment strategies in noninferiority trials, with the former representing the experimental arm(s).41 FDG-PET/CT negativity was defined as Deauville 5PS of 1 or 2; early-stage patients in both the H10F and H10U studies with negative PET-2 received chemotherapy alone versus CMT with INRT in the control (non-PET-based) treatment arm (Figure 3 and Table 2). Patients with positive PET-2 in the experimental arms of H10F and H10U had treatment intensified to BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone)-escalated and INRT. With relatively early follow-up, preplanned interim analyses were performed for H10F and H10U.
Clinical trial designs of recently completed and ongoing phase 3 randomized studies of response-adapted therapy for adult early-stage HL. (A) EORTC/LYSA/FIL H10F study. *None of the following present: large mediastinal mass, age ≥50 years, high ESR, or 4 or more areas. (B) EORTC/LYSA/FIL H10U study. *Any of the following present: large mediastinal mass, age ≥50 years, high ESR, and/or 4 or more areas. (C) UK-led RAPID study; all PET-3+ patients received a 4th cycle of ABVD followed by 30 Gy of IFRT. (D) GHSG HD16 favorable trial. *None of the following present: large mediastinal mass, extranodal disease, high ESR, or 3 or more areas. (E) GHSG HD17 unfavorable trial. *Any of the following present: large mediastinal mass, extranodal disease, high ESR, and/or 3 or more areas. High ESR for all of above defined as: >50 mm without B symptoms or ESR <30 mm with B symptoms. esc indicates escalated; ESR, erythrocyte sedimentation rate; LYSA, Lymphoma Group and the Lymphoma Study Association; FIL, Fondazione Italiana Linfomi; and pts, patients.
Clinical trial designs of recently completed and ongoing phase 3 randomized studies of response-adapted therapy for adult early-stage HL. (A) EORTC/LYSA/FIL H10F study. *None of the following present: large mediastinal mass, age ≥50 years, high ESR, or 4 or more areas. (B) EORTC/LYSA/FIL H10U study. *Any of the following present: large mediastinal mass, age ≥50 years, high ESR, and/or 4 or more areas. (C) UK-led RAPID study; all PET-3+ patients received a 4th cycle of ABVD followed by 30 Gy of IFRT. (D) GHSG HD16 favorable trial. *None of the following present: large mediastinal mass, extranodal disease, high ESR, or 3 or more areas. (E) GHSG HD17 unfavorable trial. *Any of the following present: large mediastinal mass, extranodal disease, high ESR, and/or 3 or more areas. High ESR for all of above defined as: >50 mm without B symptoms or ESR <30 mm with B symptoms. esc indicates escalated; ESR, erythrocyte sedimentation rate; LYSA, Lymphoma Group and the Lymphoma Study Association; FIL, Fondazione Italiana Linfomi; and pts, patients.
In H10F, approximately 190 patients had been randomized to each study arm; the PET-2− rate was 86%. At that point, 1 event had occurred in the INRT arm versus 9 events in the PET-based (no INRT) arm. In the H10U study, approximately 260 patients had been randomized to each study arm with a PET-2− rate of 75%; 7 events had occurred in the INRT arm versus 16 in the PET-based (no INRT) arm. Despite the low absolute number of events, statistical analyses in both H10F and H10U showed that the null hypotheses of inferiority of the experimental PET-based treatment arms would not be rejected and futility was declared for both studies (P = .017 and P = .026, respectively). In other words, if accrual continued to the original number of planned study patients, it was unlikely that equivalence would be shown between the control and experimental arms. Therefore, the data safety and monitoring committee amended the study adding INRT to all treatment arms. In addition, patient enrollment was increased in the PET+ arms to improve statistical power for the planned objectives. The study completed overall enrollment in June of 2011 with 1952 total patients; results from the interim PET+ treatment groups are awaited.
Results from the United Kingdom National Cancer Research Institute RAPID study have been presented in abstract form.42 This also was a phase 3 noninferiority randomized study that enrolled 602 patients with stage I/II nonbulky HL. All patients (ie, favorable and unfavorable groups) were included/studied in one cohort. All patients received 3 cycles of ABVD, which was followed by FDG-PET/CT (ie, PET-3); negative FDG-PET/CT was also defined by Deauville 5PS of 1 or 2 (Table 2). Patients with positive PET-3 result received an additional cycle (fourth) of ABVD followed by IFRT, whereas PET-3− patients were randomized to IFRT versus no IFRT. The study was powered to exclude ≥7% difference in PFS (lowest acceptable 3-year PFS of 88% in the no IFRT arm). Of the initial 602 patients, 571 underwent PET-3, with 75% of patients being negative.
At a median follow-up of 49 months from randomization, the 3-year PFS rates on intent to treat (ITT) for PET-3− patients who received IFRT versus not were 94.5% (91.3%, 97.7%) versus 90.8% (86.8%, 94.7%), respectively (hazard ratio = 1.51, P = NS). Notably, this 3-year absolute risk difference yielded 95% confidence intervals of 1.2% to −9.9%, with the −9.9% limit exceeding the prespecified noninferiority boundary. It is important to highlight that, a “per protocol” analysis excluded 26 patients allocated to IFRT but did not receive it and 2 patients allocated to no IFRT who received it (John Radford, personal communication). Of the 5 early deaths on study, all occurred in patients before receiving allocated IFRT. Further, all 5 of these patients were >60 years of age, with 3 deaths being due to apparent treatment-related toxicities and at least 2 due to pneumonitis. On the per protocol analysis, 3-year PFS was 97.0% for the IFRT arm compared with 90.7% for no IFRT (P = .03). This would suggest that noninferiority is not present for 3-year PFS. OS at 3 years was 97.1% in the IFRT arm and 99.5% in the no-IFRT arm (ITT analysis, P = NS). Three-year PFS and OS rates from registration for the patients with a positive PET-3 were 86.2% and 94.3%, respectively. Final analysis and publication of this study are awaited.
Noninferiority study analyses
There are several salient considerations when examining results from a noninferiority trial.43,44 In a superiority trial, ITT analysis leads to more conservative analyses and robust conclusions by reducing bias to help ensure that postrandomization circumstances (eg, noncompliance or contamination of prescribed therapy) do not confound the compared populations in a systematic way. For noninferiority studies, however, these factors have the reverse impact.44 An ITT brings the results of a comparative study closer together and may “hide” a truly inferior comparator treatment arm. Therefore, one should perform a “per protocol” analysis in noninferiority studies. Furthermore, the expectation is that the per protocol analysis yield the same result as ITT, otherwise this may lead to uncertainty and instability regarding the ultimate correct study conclusion.
Ongoing phase 3 clinical trials using response-adapted strategies in early-stage HL
The GHSG is examining the strategy of response-adapted therapy for favorable and unfavorable HL in the HD16 and HD17 noninferiority randomized trials, respectively (Table 2). HD16 (www.ClinicalTrials.gov identifier #NCT00736320) and HD17 (www.ClinicalTrials.gov identifier #NCT01356680) are similar to the EORTC design in randomizing patients to a standard non-PET-based treatment versus a PET response-adapted therapeutic strategy (ie, no IFRT with negative FDG-PET/CT), as shown in Figure 3. A notable difference in treatment is the use of BEACOPP escalated as a component of therapy in HD17. Further, the noninferiority margins for these studies are set at 5%. It may be anticipated that similar results of “inferiority” for PFS will be identified for the non-RT arms; however, the treatment groups are defined differently for GHSG versus the EORTC studies and a non-ABVD regimen is being examined for the unfavorable group in HD17. Results from these studies are eagerly awaited.
FDG-PET considerations, new imaging techniques, and novel therapeutic agents
Additional FDG-PET considerations
The results of interim FDG-PET/CT studies should be reviewed with the understanding of limitations for their generalizability and the interpretation criteria. The PPV of PET-2 in HL needs to be further improved to better guide management even after implementation of the Deauville 5PS criteria. There are data suggesting that PET-2+ patients have larger lesions after cycle 2 of therapy. In a study of 88 patients with stages I-II nonbulky HL by IHP and Deauville 5PS criteria, the percentage decrease in the sum of the products of the perpendicular diameters after 2 cycles strongly correlated with 2-year PFS.17 The combined analysis of PET-2 with CECT-2 data suggested an improvement in prediction of 2-year PFS compared with each test alone. In the PET-2+ group, a negative diagnostic CECT, defined as a decrease in the size of a mass greater than 65%, decreased the false-positive PET results. This increased the predictive value for PFS by 27%–35%, although some confidence intervals were not reliable due to small sample sizes. These findings were supported by recent data after chemotherapy in advanced-stage HL patients treated in the HD15 GHSG trial.45 In a subgroup of 54 PET+ patients after completion of chemotherapy with a reduction in tumor size of <40%, the risk of progression or relapse within the first year was 23% versus 5% for patients with a larger reduction. These results should prompt further examination of the combination of PET-2 and diagnostic CECT toward a fusion of qualitative and quantitative analyses.
New techniques
There are ongoing efforts to develop PET-based and other quantitative methodologies that measure tumor metabolic volume (MTV) or total lesion glycolysis, which may be a more accurate assessment of disease/tumor burden. In a recent study, pretreatment PET parameters MTV and maximum standardized uptake value did not correlate with outcome; however, change in MTV between interim and baseline studies was associated with median PFS (P = .01), as was maximum standardized uptake value (P = .02).46 In addition, a recent analysis examined the prognostic importance of baseline (pretreatment) total MTV in untreated HL patients.47 Baseline total MTV more accurately predicted outcome than tumor bulk and was prognostic in multivariate analysis for PFS.
Novel imaging biomarkers include measurement of tumor heterogeneity, which is emerging as an important factor in imaging analyses.48 The noninvasive assessment of tumor-proliferative activity may also provide a tool for individualized treatment. The 3′-deoxy-3′-18F-fluorothymidine (FLT) is the most extensively investigated functional imaging probe for measurement of cancer cell proliferative capacity.49 The role of FLT-PET will depend in part in its ability to predict early response during treatment, rather than determining the extent of disease involvement at staging. The clinical utility of FLT as an early response surrogate to date has been demonstrated in preliminary clinical studies in non-HL.49
Multiparametric MRI, which combines anatomic T2-weighted (T2W) imaging with dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted imaging (DWI), evaluates perfusion and diffusion characteristics, respectively.50 DCE-MRI provides assessment of tumor angiogenesis and enables the depiction of physiologic alterations and morphologic changes. A preliminary study reported improvement in detection of splenic involvement in HL when T2-weighted imaging was complemented by DCE-MRI.51 However, quantitative analysis of MRI data using DCE-MRI is still in evolution. With the advent of integrated PET/MRI platforms, the potential complementary nature of MRI and PET will undergo continued investigation.
Novel therapeutic agents
Brentuximab vedotin (BV) is an antibody drug conjugate with significant activity in patients with relapsed/refractory HL, and clinical studies are ongoing that incorporate this agent earlier in the treatment course of HL patients, including frontline. It will be important to determine the impact of FDG-PET/CT with BV alone and in combination with standard chemotherapy. There are recent FDG-PET/CT data in relapsed/refractory and newly diagnosed HL. Using Deauville 5PS as visual analysis, investigators analyzed the prognostication of interim FDG-PET/CT with single-agent BV for a small cohort of relapsed/refractory HL patients.52 After a median of 3 BV doses, 67% were interim-PET+ (5PS 4-5); 1-year PFS rates were 100% and 38%, respectively, for patients with negative and positive interim FDG-PET/CT, respectively (P = .033).
Additional FDG-PET/CT data using sequential BV followed by ICE chemotherapy before autologous stem cell transplantation have been reported53 ; the PET− rate (5PS 1-3) using concurrent BV and ABVD or AVD chemotherapy for newly diagnosed HL was 96%.54 The prognostic impact of FDG-PET/CT with incorporation of novel therapeutic agents should continue to be examined.
Conclusions
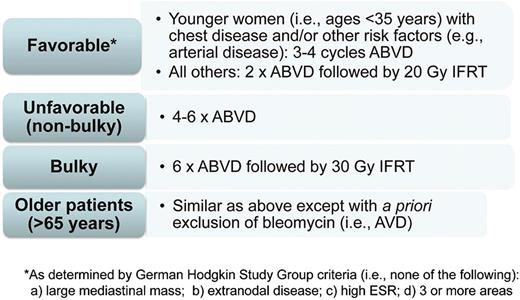
FDG-PET/CT is an important tool for clinicians in the diagnosis and management of patients with HL. Standardization of the interpretation and reproducibility of FDG-PET/CT (eg, Deauville 5PS) have been critical in the application of this imaging modality in clinical practice. Prospective and randomized clinical studies evaluating the impact of FDG-PET/CT for response-adapted approaches have been completed. In terms of the question of whether interim FDG-PET/CT is a compass for a safe navigation in HL,19 the current answer with existing techniques and available data is no. Based on present data, FDG-PET/CT has not been able to discriminate a low-risk early-stage HL group in whom RT may be withheld with respect to acute disease control. The type or modification of therapy based on interim FDG-PET/CT in early-stage HL is not advocated in routine clinical practice at this time. It should also be considered that the currently available results from response-adapted studies do not dictate that RT should be recommended for all early-stage HL patients. In part since the primary goal in treating most HL patients is long-term OS, it remains a clinical management choice for physicians and patients to make.55 Figure 4 depicts personal recommendations for the treatment of early-stage HL based on currently available data.
How I treat early-stage adult HL in 2014. Shown are the treatment strategies advocated by A.M.E. based on current clinical data. Based on available data, treatment should not be modified based on results of interim FDG-PET/CT; however, continued follow-up of ongoing studies, including results from studies examining intensification based on “positive” interim FDG-PET/CT, is needed. The treatment algorithms are separated by different early-stage subgroups: favorable, unfavorable (nonbulky), bulky, and older patients.
How I treat early-stage adult HL in 2014. Shown are the treatment strategies advocated by A.M.E. based on current clinical data. Based on available data, treatment should not be modified based on results of interim FDG-PET/CT; however, continued follow-up of ongoing studies, including results from studies examining intensification based on “positive” interim FDG-PET/CT, is needed. The treatment algorithms are separated by different early-stage subgroups: favorable, unfavorable (nonbulky), bulky, and older patients.
In addition, there should not be a rush toward final judgment regarding FDG-PET/CT response-adapted data in HL. We must await longer follow-up of reported trials. The outcomes of recently completed and ongoing FDG-PET/CT response-adapted studies in early-stage HL are eagerly awaited, especially toward potential longer-term OS differences that may be gleaned. We also await data from the treatment arms with positive interim FDG-PET/CT where treatments were escalated to more intensive therapy. Further, there are multiple important ongoing response-adapted clinical trials in advanced-stage HL, in which the prognostic impact of FDG-PET/CT is much more pronounced.9 We should also continue to explore new and novel techniques of functional imaging and innovative applications such as metabolic tumor burden/volume, tumor proliferation via FLT, and integrated PET/MRI. Finally, examination is needed of the prognostic impact of FDG-PET/CT with new/targeted therapeutic agents and the integration of biologic biomarkers in combination with functional imaging modalities to identify the most robust predictive markers of patient outcome.
This article was selected by the Blood and Hematology 2014 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2014. It is reprinted in Hematology Am Soc Hematol Educ Program. 2014;2014:135-143.
Acknowledgments
The authors thank Ralph M. Meyer (McMaster University and the Juravinski Hospital and Cancer Centre, Hamilton, Ontario, Canada) for insightful discussions and guidance.
Authorship
Contribution: A.M.E. and L.K. wrote the manuscript.
Conflict-of-interest disclosure: A.M.E. has received honoraria and research funding from Seattle Genetics, Millennium, and Celgene. Off-label drug use: brentuximab vedotin for frontline/untreated HL.
Correspondence: Andrew M. Evens, Division of Hematology-Oncology and Tufts Cancer Center, Tufts Medical Center, 800 Washington St, Boston, MA 02111; e-mail: aevens@tuftsmedicalcenter.org.