Key Points
Kogenate Bayer/Helixate NexGen was associated with a higher inhibitor incidence than Advate in 407 consecutive UK severe hemophilia A previously untreated patients.
Other risk factors for inhibitor development were factor VIII genotype, ethnicity, and intensive treatment episodes.
Abstract
The effect of recombinant factor VIII (rFVIII) brand on inhibitor development was investigated in all 407 severe hemophilia A previously untreated patients born in the United Kingdom (UK) between 1 January 2000 and 31 December 2011. Eighty-eight (22%) had been in the RODIN study. Information was extracted from the National Haemophilia Database. Because exposure days (EDs) were not known for some patients, time from first treatment was used as a surrogate for rFVIII exposure. An inhibitor developed in 118 (29%) patients, 60 high and 58 low titer, after a median (interquartile range) of 7.8 (3.3-13.5) months from first exposure and 16 (9-30) EDs. Of 128 patients treated with Kogenate Bayer/Helixate NexGen, 45 (35.2%, 95% confidence interval [CI] 27.4-43.8) developed an inhibitor compared with 42/172 (24.4%, 95% CI 18.6% to 31.4%) with Advate (P = .04). The adjusted hazard ratio (HR) (95% CI) for Kogenate Bayer/Helixate NexGen compared with Advate was 2.14 (1.12-4.10) (P = .02) for high titer and 1.75 (1.11-2.76) (P = .02) for all inhibitors. When excluding UK-RODIN patients, the adjusted HR (95% CI) for high-titer inhibitors was 2.00 (0.93-4.34) (P = .08). ReFacto AF was associated with a higher incidence of all, but not high-titer, inhibitors than Advate. These results will help inform debate around the relative immunogenicity and use of rFVIII brands.
Introduction
The Research Of Determinants of INhibitor development (RODIN) group reported that a second-generation full-length recombinant factor VIII (rFVIII) (Kogenate Bayer/Helixate NexGen; Bayer AG, Bayer Healthcare, Berkeley, CA) was associated with an increased risk of inhibitor development in previously untreated patients (PUPs) compared with a third-generation full-length rFVIII (Advate; Baxter International, Thousand Oaks, CA). The adjusted hazard ratio (HR) (95% confidence interval [CI]) was 1.60 (1.08-2.37) for all inhibitors and 1.79 (1.09–2.94) for high-titer inhibitors.1 The authors discussed whether this association “may be a biased finding (through confounding, selection or information bias), or may be a chance finding, or a causal effect.” They argued at the time1 and later2 that bias was an unlikely explanation for the observation. Other experts have raised questions relating to study methodology and analysis,3-5 but these have been defended by the authors.2
Previous publications, including the Kogenate Bayer licensing studies and a systematic review, had suggested that the inhibitor risk associated with Kogenate Bayer might be lower than average,6,7 although the inclusion of minimally treated patients in the licensing study may have influenced this observation. The European Medicines Agency (EMA) reviewed the available evidence and concluded on 6 December 2013 that “current evidence does not confirm increased risk of inhibitor development compared with other factor VIII products” (EMA/741427/2013; http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Kogenate_Bayer_Helixate_Nexgen_20/Recommendation_provided_by_Pharmacovigilance_Risk_Assessment_Committee/WC500157065.pdf).
Further studies are needed to provide additional information about the incidence of inhibitor development in PUPs associated with different rFVIII brands. The United Kingdom (UK) Haemophilia Centre Doctors’ Organization (UKHCDO) therefore investigated the risk factors, including brand of rFVIII, for inhibitor formation in all patients with severe hemophilia A born in the UK between 1 January 2000 and 31 December 2011.
Methods
Data reported to the UKHCDO National Haemophilia Database (NHD) were analyzed under an agreement with the UK Data Protection Registrar. The following data were extracted from the NHD or requested to be reported to the NHD from all UK Haemophilia Centres: date of birth, ethnicity, family history (FH) of hemophilia, FH of a FVIII inhibitor in any relative, FVIII mutation, date of first FVIII treatment, brand of FVIII first infused, intensive treatment event at first exposure or during the first 50 exposure days (EDs) but before inhibitor detection (intensive treatment after an inhibitor had developed was excluded), units of FVIII used annually prior to 2005 or quarterly after 2005, date of inhibitor diagnosis, peak inhibitor titer, and EDs prior to inhibitor formation. Data on the rFVIII usage and time from first treatment to reach 75 EDs were known for 50 noninhibitor patients entered into RODIN and were compared with rFVIII usage in the noninhibitor patients in the rest of the cohort.
Ethnicity is reported as white or nonwhite; FVIII mutations were categorized as high risk (large deletions, nonsense mutations, intron 1 and 22 inversions), low risk (small deletions and insertions, missense mutations, splice site mutations), or unknown (no mutation detected or unknown).8 This definition of high-risk/low-risk genotype replicates that used in RODIN. Inhibitor titres were measured locally using standard Bethesda or Nijmegen assays. All laboratories participated in national external quality-control schemes. High-titer inhibitors were categorized as ≥5 BU and low titer as <5 BU. Intensive treatment was 5 or more consecutive EDs.
The frequency of inhibitor testing was at the discretion of the local clinician. Although guidelines recommended testing every fifth ED until 20 EDs and then less frequently,9,10 the actual frequency was not reported.
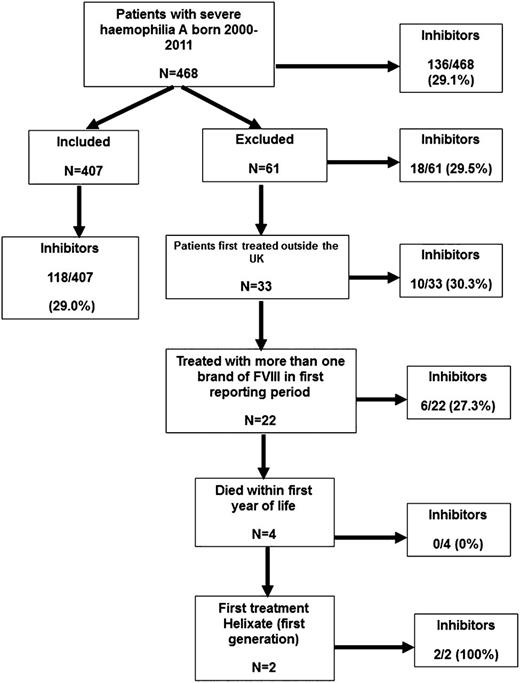
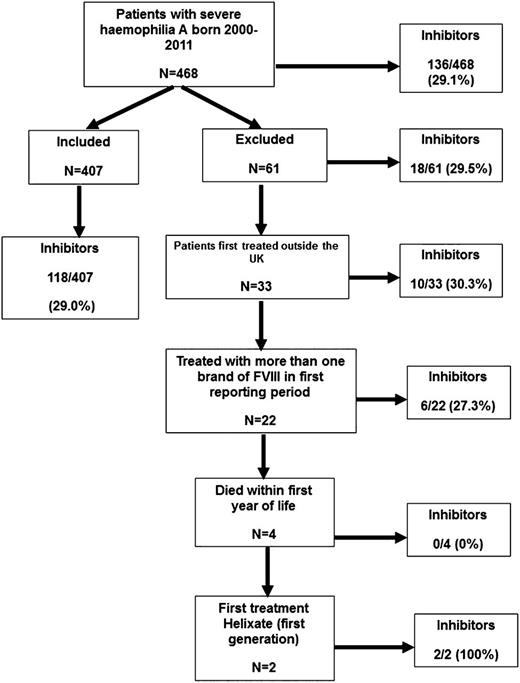
Brand of rFVIII was recorded annually throughout the study. From 2005, the total amount of each brand infused by each patient was collected, first annually and then, from 2007, quarterly. Clinical trial participants were included. Immigrants to the UK were excluded because their full treatment history was not known. Patients who had received >1 brand of rFVIII in the first year of treatment were excluded because it was not known which agent had been used first. Patients who died in their first year of life were excluded because of insufficient follow-up, and 2 patients treated with Helixate (first generation) were excluded because there were too few cases to analyze (Figure 1). Inhibitors were detected by surveillance or clinical presentation.
Patients were followed until (1) they changed rFVIII brand, (2) they developed an inhibitor, (3) they moved abroad, or (4) 30 June 2013. No patients who remained in the UK were lost to follow-up. If the actual date of an event was unknown, it was assumed to have occurred at the midpoint of the relevant reporting period.
Statistical analysis
Data were described using median, interquartile range and range, and number and percentage. Comparisons between groups were made using Mann Whitney U, Kruskal-Wallis, χ2, and Fisher’s exact tests, as appropriate. HRs describing the risk of inhibitor development were calculated by fitting univariate and multivariate proportional hazards models, using length of follow-up as the time variable. Multivariate models adjusted for ethnicity, FVIII mutation, age at first exposure to FVIII, FH of hemophilia, FH of inhibitors, intensive treatment at first treatment, year of first treatment, and treatment center (the 10 centers with ≥5 inhibitors [total 80 inhibitors] were entered individually and the other centers [total 38 inhibitors] were pooled as 1 center). Intensive treatment after first exposure was excluded from the model because it could not be included in a time-dependent manner. Missing data for these covariates were imputed using multiple logistic regression models. Patients in the RODIN study were included in the main analysis but were also the subject of subset analyses. Time to inhibitor development after first treatment is shown in Kaplan-Meier plots, and different brands of rFVIII were compared by log-rank test.
Results
Patient population
A total of 468 severe hemophilia A PUPs were born between 1 January 2000 and 31 December 2011; 61 were excluded for reasons shown in Figure 1. The incidence of inhibitors in the 407 patients in the main analysis and the 61 patients excluded was similar (118/407 [29%] and 18/61 [29.5%]).
Of the 118 inhibitors in the main analysis, 60/407 (14.7%) were high titer and 58/407 (14.3%) low titer. The median (interquartile range [IQR]) peak titer was 5.0 (1.2-27.6) BU diagnosed after 16 (9-30) EDs. The time to inhibitor diagnosis was 7.8 (3.3-13.5) months after first treatment. The noninhibitor patients were followed for 45 (24-61.7) months (Table 1). PUPs were treated in 51 centers, and the median (IQR) number of inhibitors/center (in centers reporting ≥1 inhibitor, n = 27) was 3 (2-5). Four centers had entered 88 patients into RODIN (22% of the UK cohort). The RODIN centers were involved in the treatment of an additional 56 PUPs, often following referral for inhibitor treatment or through shared care arrangements, and hence could not have been recruited to RODIN.
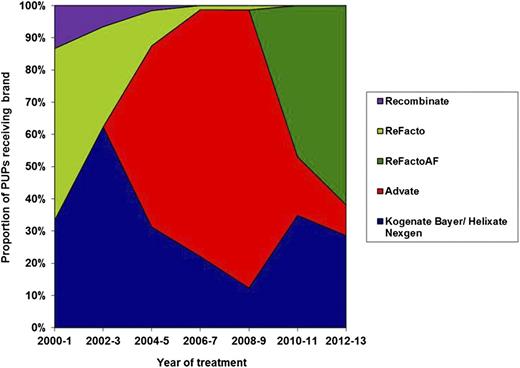
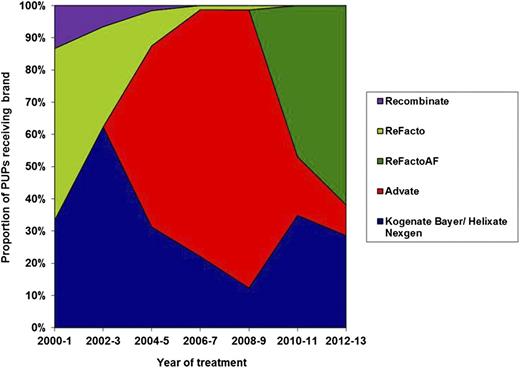
Of the 407 PUPs, 172 were first treated with Advate, 128 with Kogenate Bayer or Helixate NexGen, 52 with ReFacto, 44 with ReFacto AF, and 11 with Recombinate. The proportion of patients treated with each brand of rFVIII by date of first treatment is shown in Figure 2.
Proportion of PUPs treated with each brand of rFVIII grouped in 2-year bands. The proportion of patients treated with each brand of FVIII changed through the time of study due to the launch of new products, national tenders, and clinician and patient choice.
Proportion of PUPs treated with each brand of rFVIII grouped in 2-year bands. The proportion of patients treated with each brand of FVIII changed through the time of study due to the launch of new products, national tenders, and clinician and patient choice.
The characteristics of the study subjects and their risk factors for inhibitor development, by first brand of rFVIII, are shown in Table 1. Overall, 60% of patients had a high-risk FVIII mutation. Of the 232 patients with an FH of hemophilia, 41 (17.7%) had an FH of an inhibitor. An intensive treatment occurred in 13.8% at first exposure and 43% subsequently within the first 50 EDs and before inhibitor development (Table 1).
Factor VIII exposure
The 50 inhibitor-free RODIN patients for whom data were available took a median (IQR) 13.3 (7.9-19.1) months from first treatment and used 36 950 (24 187.5-72 312.5) units of FVIII before reaching 75 EDs. Of these, 32/50 treated with Advate took 12.4 (7.5-18.6) months and used 36 950 (21687.5-71437.5) units to reach 75 EDs. The 10 patients treated with Kogenate took 17.0 (13.7-22.7) months and used 28 875 (19312.5-75375) units.
Median (IQR) follow up for inhibitor-negative patients was 45 (24-62) months. Patients treated with Advate were followed for median of 49.5 months (until change of brand or study end) compared with 36, 59.2, 18, and 12 months for Kogenate Bayer/Helixate NexGen, ReFacto, ReFacto AF, and Recombinate, respectively (Table 1). Of the 234 non-RODIN inhibitor-free patients, 220 (94%) were followed for ≥13.3 months and 209 (89.3%) for ≥19.1 months (all brands of rFVIII). Of the 158 non-RODIN inhibitor-free patients first treated in 2005 or later, 128 (81.0%) had received at least 36 950 IU and 117(74.1%) at least 72 312.5 IU of rFVIII (all brands of rFVIII).
In the 45 inhibitor patients treated with Kogenate Bayer/Helixate NexGen (for whom data were available), the inhibitor was diagnosed after 15 (8-31) EDs (n = 43) taking 9.0 (5.7-13.3) (n = 45) months after first treatment. In the 42 Advate inhibitor patients (data on n = 40), diagnosis was after 17.5 (10-28) EDs taking 7.4 (3.4-13.0) months.
Proportion of patients developing an inhibitor over time
There was a trend for the incidence of low-titer inhibitors to increase and high-titer inhibitors to decrease with time. Between 2000 and 2006, there were 25 low-titer inhibitors in 205 PUPs (12.2%) compared with 33/202 (16.3%) during 2007-2013 (P = .23). In 2000-2006, there were 37/205 high-titer inhibitors (18.1%) compared with 23/202 (11.4%) in 2007-2013 (P = .06).
Investigation of risk factors for FVIII inhibitor formation
Table 2 shows the effect of previously reported risk factors for inhibitor development in this cohort. High-titer inhibitors were associated with nonwhite ethnicity and high-risk mutations. All inhibitors were associated with intensive treatment at first exposure and high-risk mutations.
The effect of brand of rFVIII on the incidence of inhibitor development
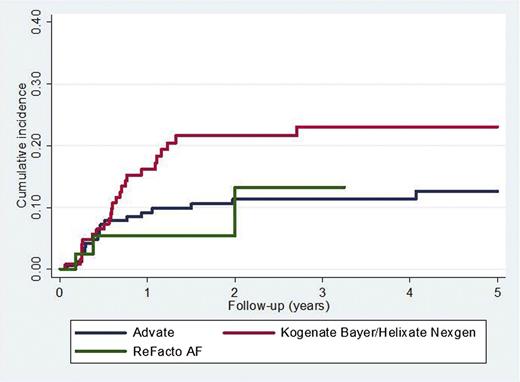
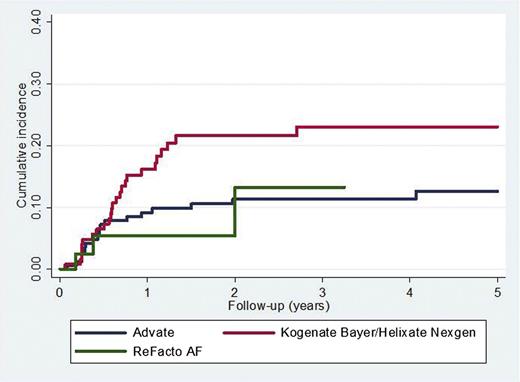
The association between rFVIII brand and the incidence of inhibitor development is shown in Table 3. There were 42 inhibitors reported in 172 (24.4%) patients first treated with Advate and 45/128 (35.2%) treated with Kogenate Bayer/Helixate NexGen (P = .04). The adjusted HR (95% CI) for Kogenate Bayer/Helixate NexGen compared with Advate was 2.14 (1.12-4.10) (P = .02) for high-titer inhibitors (Figure 3) and 1.75 (1.11-2.76) (P = .02) for all inhibitors. If intron 1 and 22 inversions were not categorized as high-risk mutations, the results were virtually unchanged (data not shown).
Time to high-titer inhibitor diagnosis comparing Kogenate Bayer/Helixate NexGen and Advate.P = .02.
Time to high-titer inhibitor diagnosis comparing Kogenate Bayer/Helixate NexGen and Advate.P = .02.
ReFacto AF was associated with an increased incidence of all inhibitors compared with Advate. There were 15 inhibitors in 44 patients (34%) treated with ReFacto AF compared with Advate, with an adjusted HR (95% CI) of 2.63 (1.26-5.47) (P = .01). The incidence of high-titer inhibitors did not differ between Advate and ReFacto AF (Table 3). The incidence of inhibitor formation over the time of the study is shown in Table 4. The increased incidence of inhibitor formation associated with Kogenate Bayer/Helixate NexGen compared with Advate was found during the first two 4-year time periods, but not the last 4 years (Table 4).
A comparison was made between UK-RODIN and UK-non-RODIN subjects (Table 5). UK-RODIN subjects had a higher proportion of inhibitors than non-RODIN (33/88 [37.5%] vs 85/319 [26.7%]; P = .05). In the UK-RODIN subjects, 17 (19.3%) had high-titer and 16 (18.2%) low-titer inhibitors compared with 43 (13.5%) high-titer and 42 (13.2%) low-titer inhibitors in non-RODIN subjects. A comparison of risk factors between UK-RODIN and non-RODIN subjects (Table 5) showed that UK-RODIN patients were more likely to have had an intensive treatment at first exposure (P = .02) and an FH of inhibitors (P = .03). The number of patients with an FH of inhibitors who received Kogenate/Bayer Helixate NexGen in the UK-RODIN and non-RODIN groups was 5 and 13, respectively, and for Advate 13 and 9, respectively.
When the UK-RODIN and non-RODIN subjects were analyzed separately, Kogenate Bayer/Helixate NexGen was not associated with an increased risk of high-titer inhibitors in UK-RODIN subjects, with an adjusted HR (95% CI) of 2.90 (0.49-17.13) (P = .24). In non-RODIN subjects, the adjusted HR was 2.00 (0.93-4.34) (P = .08). There was an increased incidence of all inhibitors in the UK-RODIN group (P = .02) and a trend toward an increase in the non-RODIN group (P = .08) for Kogenate Bayer/Helixate NexGen compared with Advate. The number of subjects and inhibitor events included in these analyses was low, especially for the RODIN group (Table 3).
If participation in UK-RODIN was included in the multivariate analysis, the adjusted HR (95% CI) with Kogenate Bayer/Helixate NexGen compared with Advate was 1.82 (1.15-2.89) (P = .01) for all and 2.36 (1.23-4.56) (P = .01) for high-titer inhibitors.
Discussion
This study reports that, in a consecutive cohort of UK PUPs born over a 12-year period, there was a significantly higher incidence of high-titer and all inhibitors associated with Kogenate Bayer/Helixate NexGen than with Advate. This was found in both univariate and multivariate analyses. These results are similar to those reported by RODIN. There was an increased incidence of all, but not high-titer, inhibitors with ReFacto AF compared with Advate.
The strengths of the study are that the prespecified aim was to investigate the effect of rFVIII brand on inhibitor development and that it is of a complete, consecutive national cohort over a long period, minimizing the potential for selection and reporting bias. Only patients born and first exposed to rFVIII in the UK were included, because the treatment history and prior inhibitor status of patients moving to the UK was not known with confidence. It is not possible for people with severe hemophilia to receive treatment in the UK without information being reported to the NHD, and so we are confident that this is a complete national cohort. However, the study was not randomized.
Although it is unlikely that high-titer inhibitors were missed, some transient, low-titer inhibitors are likely to have escaped detection. The number of inhibitors reported (118/407 [29.0%]) is consistent with the literature11-14 and similar to the RODIN study (177/574 [30.8%]).1 The RODIN study reported a higher proportion of high-titer inhibitors than the UK study (20.2% vs 14.5%), suggesting that the cohorts may not be directly comparable. The UK study reported a higher proportion of low-titer inhibitors (14.3% vs 10.6%), suggesting that UK surveillance was at least as intensive. The number of EDs prior to inhibitor diagnosis was similar in the RODIN and UK studies (median 15 and 16 days, respectively), also suggesting similar intensities of surveillance.
All UK patients were included, irrespective of the institution they first attended, and continued to be followed continuously, even if they changed center, because their treatment and inhibitor status was known throughout. In contrast, the RODIN study reported on selected patients attending specialist centers. Patients referred because of inhibitor development were excluded from RODIN, and others were excluded for a variety of reasons. The characteristics of our patients and inhibitor rates are consistent with the literature, and similar independent risk factors for inhibitor development were identified (FVIII genotype, intensive treatment at first exposure, and ethnicity).8,11,12,15-17 The adjusted association between inhibitor development and FH of inhibitor was not significant, perhaps because this was not restricted to first-degree FH. The UK cohort is therefore a highly plausible and representative cohort of PUPs.
In our study, EDs are not known in the noninhibitor, non-RODIN patients. EMA guidance is to investigate inhibitor development in PUPs during the first 50 EDs (EMA/CHMP/BPWP/144533/2009; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109692.pdf) to standardize FVIII exposure and because most events occur during that time. Exposure to rFVIII can only be estimated in our study using time after first treatment as a surrogate and, for patients treated from 2005, quarterly rFVIII usage. Analysis of the UK-RODIN patients showed that 75 EDs were associated with a median time of 13.1 months from first treatment and the use of 38 000 IU FVIII/patient. The NHD does not have information on the time taken or the amount of rFVIII infused to reach 50 EDs in this cohort. Although time is not the best method for assessing FVIII exposure, longer follow-up of severely affected patients is almost inevitably associated with increased exposure. The median follow-up of our noninhibitor patients was 45 months, and 75% had at least 24 months follow-up. A large majority of these patients will have received >50 EDs, but the number of noninhibitor patients followed for ≤50 EDs and who remained at risk of inhibitor development is not known. The median follow-up for noninhibitor patients was longer for patients treated with Advate (49.5 months) than those treated with Kogenate Bayer/Helixate NexGen (36 months), implying that if lack of follow-up is an issue, it would have affected Advate at least as much as Kogenate Bayer/Helixate NexGen. Furthermore, our study confirmed previously described risk factors for inhibitor development in PUPs, suggesting that using time as a surrogate for exposure is a reasonable approximation.
We have assumed that time after first treatment equated to a similar rFVIII exposure, irrespective of brand. Although we cannot be certain that this is correct, there is no plausible reason why rFVIII brand should affect prescribing. In the UK-RODIN cohort, patients treated with Advate reached their 75th ED sooner and received more FVIII than those treated with Kogenate Bayer/Helixate NexGen. In the inhibitor patients, the time and number of EDs to inhibitor development was similar for Advate and Kogenate Bayer/Helixate NexGen. This supports the contention that reduced exposure to Advate compared with Kogenate Bayer/Helixate NexGen is an unlikely explanation for our findings.
It is important to recognize that 22% of the UK cohort participated in RODIN, and it was therefore necessary to investigate the impact of this subgroup on the findings. UK-RODIN patients had a higher incidence of inhibitors than the non-RODIN cohort, suggesting these groups represent different populations. This is unsurprising because RODIN subjects came from 4 specialist pediatric referral centers and, unlike the UK study, not all patients from those centers could be included in RODIN. There is a clear distinction between UK-RODIN and non-RODIN patients as described; it is difficult, however, to make a comparison between UK RODIN and UK non-RODIN centers because of the shared care arrangements. The UK-RODIN group were more likely to have had an FH of inhibitors and to have received an intensive first treatment, potentially explaining the higher incidence of inhibitors. It is possible that the RODIN centers had clusters of families prone to inhibitors because of FH. We are unable to assess this definitively because we have too few subjects available, and it remains a possible influence on our results and those of the RODIN study. Adjusting for RODIN participation in the multivariate analysis as a surrogate marker of an unidentified risk factor did not change the association of inhibitor development with brand of rFVIII. When analyzing non-RODIN patients separately, a trend toward an increase in both high-titer and all inhibitors was observed with Kogenate/Bayer/Helixate NexGen compared with Advate, demonstrating that the results in the whole UK cohort were not wholly dependent on the UK-RODIN group.
The analyses involving ReFacto (n = 52) and ReFacto AF (n = 44) are less secure, because fewer patients were studied. ReFacto AF was associated with an increased incidence of all inhibitors, whereas the incidence of high-titer inhibitors was not increased compared with Advate. There was no difference in the incidence of high-titer or all inhibitors with ReFacto compared with Advate. ReFacto AF replaced ReFacto in the UK in 2010, and a possible reason for these findings is that the intensity of inhibitor surveillance increased over time. This hypothesis is supported, in part, by the finding of a trend toward a higher incidence of low-titer inhibitors later in the study, although this is unlikely to account for all the observed difference. RODIN did not report on ReFacto AF because of very low numbers. Further data are required to assess whether ReFacto AF is associated with an altered incidence of inhibitors.
The Recombinate results are uninterpretable due to the very low numbers (n = 11) but have been reported for transparency. These findings were not seen in the RODIN study with much larger numbers.1
The RODIN methodology and interpretation have been questioned,3-5 and some of these criticisms apply to this study. Central laboratory monitoring was not used, but this would affect all rFVIII brands equally. Surveillance for inhibitors was at the discretion of the treating clinician, and this may have resulted in low-titer inhibitors being diagnosed more often for brands released later in the follow-up period.13 Whether a prospective or retrospective design is important has been debated.2,4 The UK data are analyzed retrospectively, but collection was prospective and the same national cohort would have been included whether studied prospectively or retrospectively.
A potential cause of bias could be changing practices during the 12-year period, which may have included earlier use of prophylaxis or avoidance of intensive treatment by deferring or not placing central venous catheters, although it is not known whether this happened. This may be important, because proportionately more Kogenate Bayer/Helixate NexGen was prescribed in the earlier rather than latter part of the study (Advate was launched in 2004 and ReFacto in 2010). An increased incidence of inhibitors associated with Kogenate Bayer/Helixate NexGen was observed in the first two 4-year time periods of the study, but not the last. The significance of this finding is difficult to interpret but has been included for transparency. To address this, the multivariate analysis was adjusted for year of first treatment, and this did not alter the findings.
It has been suggested that one brand of rFVIII might have been preferentially prescribed to patients with a perceived higher risk of an inhibitor,4 but this does not seem to have occurred in the UK because patient characteristics were similar between brands. The potential importance of a center effect has been raised,4 because individual clinicians may have started prophylaxis at different times, been more likely to prescribe intensive treatment, or had individual reasons for choosing a concentrate. In our analysis, we have adjusted for centers reporting ≥5 inhibitors, and this did not alter the findings.
A French study has reported an increased incidence of any inhibitor with Kogenate Bayer/Helixate NexGen compared with Advate on univariate analysis, although the association was not statistically significant after adjustment for known risk factors for inhibitor formation. There was no statistically significant increase in high-titer inhibitors, although a trend was observed. Data on ReFacto AF were not reported.18
A biologically plausible explanation for the observation of the different incidence of inhibitors is lacking, although the RODIN group argues that this does not undermine the validity their finding.2 It has been suggested that the increased inhibitor risk may relate to FVIII aggregates,2 but this is unsubstantiated by published observations. An alternative interpretation of the results may be that Advate has a lower than expected incidence of inhibitor development, although again a plausible explanation for this is lacking. In view of this, it would not be appropriate to extrapolate our findings to other brands of rFVIII, even if manufacturing processes were similar.
Postmarketing surveillance for inhibitor development in large, well-characterized, unselected cohorts of patients is important if subtle differences in immunogenicity are to be identified.14 Clinical studies are almost invariably underpowered and may not be representative of everyday practice, for example, if minimally treated patients are included, intensive treatments minimized, or prophylaxis standardized. Most studies investigate a single brand of FVIII, and comparisons between brands are not possible. Our study, together with the RODIN, French,18 and EUHASS studies,1,14 highlights the important patient safety implications of large cohorts followed over prolonged periods of time.
Our findings and those of RODIN are limited to PUPS, and there is no convincing evidence in the literature that inhibitor risk differs between products when used in previously treated patients (PTPs). The immunologic mechanisms for inhibitor development in PTPs are not well understood and likely to differ between PTPs and PUPs.
These findings support the RODIN observation of an increased incidence of inhibitor development in PUPs associated with Kogenate Bayer/Helixate NexGen compared with Advate. An increased incidence of all inhibitors associated with ReFacto AF was also observed, but this may be related to increased surveillance detecting more transient low-titer inhibitors, and the findings are less reliable due to the small numbers studied. UKHCDO has placed these observations in the public domain to help inform the debate about the relative immunogenicity of different brands of rFVIII and to highlight the need for long-term studies on large cohorts of patients independent of commercially sponsored studies. Despite any shortcomings of the RODIN, French, and UK studies, the similarity of the results for Kogenate Bayer/Helixate NexGen compared with Advate makes these findings more plausible. In conclusion, although an increased incidence of inhibitor development in PUPs associated with Kogenate Bayer/Helixate NexGen has not been definitively proven, we suggest that clinicians carefully consider these findings when prescribing rFVIII to PUPs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of all UK hemophilia centers for their hard work in collecting data and responding to data queries. The staff at the National Haemophilia Database are also thanked for their contribution to data collection. The authors thank Dr M. Soucie, Dr G. Dolan, Dr D. Keeling, Professor M. Laffan, and the UKHCDO Advisory Committee for extensive discussions and comments while the manuscript was being developed.
The study was supported by the UKHCDO and received no external source of funding.
Authorship
Contribution: P.W.C. designed the study, coordinated data collection, interpreted data, and jointly wrote the first draft of the manuscript; B.P.P. coordinated data collection, performed the analysis and revised the manuscript; E.C., D.P.H., R.L., S.R., K.T., and M.W. designed the study, interpreted data, and revised the manuscript; and C.R.M.H. designed the study, coordinated data collection, interpreted data, and jointly wrote the first draft of the manuscript.
Conflict-of-interest disclosure: P.W.C. has acted as a paid consultant to Baxter, Bayer, CSL Behring, and Novo Nordisk and received honoraria for speaking at symposia sponsored by Baxter, Bayer, CSL Behring, and Novo Nordisk. E.C. has received honoraria and education grants from Baxter and Novo Nordisk. D.P.H. has received unrestricted research grants from Bayer and Octapharma. He has also received speaking honoraria, consultancy honoraria, and/or travel grants from Bayer, Baxter, Novo Nordisk, and Pfizer. R.L. has acted as a paid consultant to Baxter, Bayer, Novo Nordisk, Pfizer, CSL Behring, and BPL and received honoraria for speaking at symposia sponsored by Bayer and Pfizer. S.R. has received speaker fees from Baxter, Grifols, and Biotest and research grants from Pfizer, Baxter, and Grifols. K.T. has received sponsorship to attend scientific meetings from Pfizer, Novo Nordisk, Bayer, and CSL Behring and consultancy honoraria from Bayer. M.W. has acted as paid consultant to Baxter, Novo Nordisk, and CSL Behring and has received sponsorship from Baxter, Bayer, and Novo Nordisk to attend scientific meetings. C.R.M.H. has acted as a paid consultant for Baxter, Novo Nordisk, and Pfizer and has received speaker honoraria from Baxter, Bayer, Novo Nordisk, Sobi, and Pfizer. B.P.P. declares no competing financial interests.
Correspondence: Peter Collins, Department of Haematology, University Hospital of Wales, Heath Park Cardiff, Cardiff CF14 4XN, UK; e-mail: peter.collins@wales.nhs.uk.