To the editor:
Eculizumab is a monoclonal antibody (mAb) reputed to block C5 by preventing enzymatic generation of active components C5a and C5b.1 C5a is a proinflammatory mediator whereas C5b combines with C6, C7, C8, and C9 to form the membrane attack complex C5b-9, which contributes to procoagulant effects and cell lysis. Eculizumab has revolutionized treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome (aHUS),2,3 thus improving the hemolysis and thrombocytopenia seen in these disorders. Clinical studies of eculizumab efficacy in vivo have relied on parameters such as platelet count or lactate dehydrogenase (LDH) levels.2,3 Ex vivo studies rely on complement hemolytic assays, which test the functional capability of serum complement components to lyse sheep or chicken erythrocytes precoated with anti-sheep or anti-chicken red cell antibody, respectively.1 It is poorly understood whether such parameters reflect C5a and C5b-9 blockade by eculizumab in vivo.
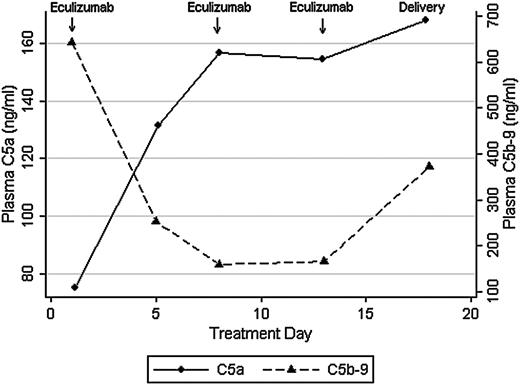
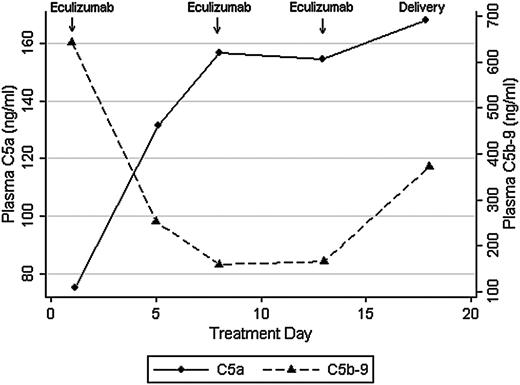
We recently used eculizumab to treat a patient with the acquired pregnancy condition of hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome, and the clinical description of this single case has been published.4 HELLP syndrome shares complement gene mutations with aHUS,5 and both conditions are characterized by elevated C5a and C5b-9 levels.6,7 In our prior report, we found that eculizumab reduced hemolysis and normalized platelet counts in HELLP syndrome, as seen with aHUS. Eculizumab was dosed at 1200 mg intravenously on days 1, 7, and 13 during hospital observation for HELLP syndrome. In this report, we sought to confirm that eculizumab treatment correlates with complement blockade, as measured by C5a and C5b-9 levels, by using enzyme-linked immunosorbent assays (ELISAs) (BD Biosciences, San Jose, CA). Approval was obtained from the Brigham and Women's Hospital institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. Despite therapeutic eculizumab levels (>100 μg/mL; more than threefold molar excess) and 100% inhibition of chicken red cell lysis by maternal serum (Alexion Pharmaceuticals), plasma C5a levels increased after treatment. After 1 week of eculizumab, and in conjunction with HELLP syndrome resolution, plasma C5a levels increased from 75.2 to 157 ng/mL whereas C5b-9 levels decreased from 644 to 160 ng/mL (Figure 1). This finding was in contrast to the hypothesis that eculizumab would effectively reduce both C5a and C5b-9 levels. C5b-9 blockade was also confirmed in urine, in which C5b-9 levels became undetectable by day 5 of treatment (13.2 to 0 ng/mL).
Plasma C5a and C5b-9 levels in response to eculizumab in HELLP syndrome.
In this single case, eculizumab effectively blocked hemolysis in maternal serum, as confirmed through clinical parameters (LDH, haptoglobin) and ex vivo assays (sensitized chicken red cell lysis). However, we were surprised to find that eculizumab blocked generation of C5b-9 but not C5a in vivo. The relevance of increased C5a levels is unclear, and we could not find literature on C5a levels in vivo following eculizumab treatment. However, discordance between complement inhibition in vivo and in vitro has also been seen with C5 blocker pexelizumab.8 In a randomized control trial that assessed C5 blockade in acute myocardial infarction, pexelizumab blunted generation of C5a but not C5b-9. In contrast, pexelizumab blocked both complement components in cell culture. This observation suggests that production and clearance of C5a and C5b-9 in vivo may be disease and tissue specific, and that the effects of C5 blockade are likely drug specific.
Preceding the development of eculizumab, experiments with a single-chain variable fragment (scFV) C5 antibody (using the N19-8 mAb epitope) were hindered by lack of C5a inhibition.9 It was evident that N19-8 scFV did not bind precisely to C5 regions recognized by C5 convertases but instead blocked convertase interaction through steric hindrance. Investigators noted that the N19-8 mAb epitope for C5 was on the β-chain, but the C5 cleavage site was on the α-chain. There is also a thrombin-specific cleavage site on the α-chain of C5 (R947) that is distinct from standard C5 convertases (R751).10 Cleavage at R947 generates C5a and thrombin-specific C5b. Taken together, these observations suggest that C5a may be generated by serine proteases (eg, thrombin) extrinsic to the complement cascade and that enzymatic generation of C5a does not necessarily imply symmetric (1:1) formation of the terminal complement complex C5b-9.
Eculizumab, a humanized (5G1.1) mAb, was designed to overcome partial C5 inhibition that hindered previous compounds. However, the effects of eculizumab on C5a generation have been primarily assessed ex vivo by using hemolytic assays with chicken red blood cells.1 It is unclear whether C5a generation is equally mitigated in vivo, particularly in light of antibody independent and extrinsic complement activation. In this case of HELLP syndrome, we found that eculizumab effectively blocks generation of C5b-9 but not C5a in vivo. Plasma C5a levels increased during eculizumab treatment and, although the clinical picture improved initially, HELLP syndrome eventually recurred. The ELISA that we and others have used measures C5a and C5a(desArg). C5a is a 74-amino-acid polypeptide that is rapidly metabolized in vivo to C5a(desArg), which lacks the C-terminal arginine. It is possible that we are recognizing a shift toward C5a(desArg), which is less potent than C5a and has a longer half-life. However, during the development of the humanized (5G1.1) mAb (eculizumab), investigators also used an ELISA that measured C5a/C5a(desArg).1,11 Regardless, we suspect that eculizumab blocks generation of C5a in sensitized red cell assays (ex vivo) but may not prevent enzymatic generation of C5a in vivo. Although C5 blockade has proven efficacious in a variety of clinical disorders, further research is warranted to assess the potential benefits or harms of C5a generation following treatment with eculizumab.
Authorship
Contribution: R.M.B. and B.B.F. designed the research; R.M.B. performed experiments; and R.M.B., N.R.B., and B.B.F. analyzed results and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard M. Burwick, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd, Mail Code L-458, Portland, OR 97239; e-mail: burwick@ohsu.edu.