Abstract
Invasive fungal diseases (IFDs) represent an important cause of treatment failure in adults with acute leukemia. Because of leukemia’s heterogeneity, the risk for IFDs is highly variable. We therefore apply a risk-adapted antifungal strategy with strong emphasis on pretreatment and day-15 posttreatment to allow earlier and more individualized interventions. We determine pretreatment risks for IFDs based on 4 factors: (1) host fitness for standard therapy (ie, fit, unfit, or frail); (2) leukemia resistance (high vs low probability of achieving complete remission [CR]); (3) anticipated treatment-related toxicity such as neutropenia, mucositis, and steroid-induced immunosuppression; and (4) patient exposure to opportunistic fungi. Accordingly, we stratify patients as high, intermediate, or low risk for IFDs and apply risk-adapted antifungal strategies, including primary or secondary prophylaxis and diagnostic-based preemptive or empiric therapy. Prevention of IFDs also relies on optimizing organ function, decreasing exposure to opportunistic fungi, and improving net state of immunosuppression with use of better-tolerated and investigational agents for unfit patients and those with adverse leukemia biology. Novel targeted and safe therapies that can achieve higher rates of sustained CR among patients with adverse genetics offer the best promise for reducing the burden of IFDs in these patients.
Introduction
The outcome of adults with acute leukemia (A-Leuk), including acute myelogenous leukemia (AML) and acute lymphocytic leukemia (ALL), has improved over the last decade because of the availability of novel agents and improvements in supportive care such as effective antifungal prophylaxis.1-3
Despite these advances,1-3 treatment failure remains common and is driven by adverse leukemia genetics (translated into lower probability of achieving complete remission [CR])4-6 and by early treatment-related mortality (e-TRM,7 most commonly caused by infections, particularly invasive fungal diseases [IFDs]), which serve as markers of poor host fitness for therapy.8
We herein discuss novel risk-adapted and dynamic strategies for the prevention, diagnosis, and treatment of IFDs in patients with A-Leuk, with strong emphasis on pretreatment parameters that allow earlier interventions.
Fungal infections in children and recipients of hematopoietic cell transplantation and those caused by Pneumocystis jiroveci are not discussed. The following clinical course of a patient illustrates some pitfalls in managing IFDs in patients with A-Leuk.
A 45-year-old man was hospitalized for remission-induction of favorable cytogenetics AML using cytarabine and idarubicin (7 + 3). Prophylactic fluconazole was started along with thrice-weekly serum Aspergillus galactomannan index (s-GMI; positive if ≥0.5). On day +5, the patient developed blood culture–negative febrile neutropenia (FN) of 101.5°F with an absolute neutrophil count (ANC) of <100 μL. Piperacillin-tazobactam was started with rapid defervescence. On day +10, low-grade 99.5°F fevers were noted. Physical examination was normal, but s-GMI was elevated at 0.630, rising to 1.735 on day +12. Computed tomography of the chest (chest CT) revealed a 0.4-cm nodule and tree-in-bud pattern infiltrates in the right upper lobe, prompting IV voriconazole for invasive pulmonary aspergillosis (IPA). On day +15, s-GMI reached 2.134, then normalized on day +19 when ANC reached 350/μL. Day +16 bone marrow biopsy showed no blasts. The patient’s clinical course and serial s-GMI remained unremarkable until day +23 when he complained of cough and dyspnea on exertion with hypoxemia. Chest CT revealed marked right upper lobe worsening with wedge-shaped infiltrates. A day +21 sputum yielded Aspergillus versicolor. Because clinical and radiologic worsening coincided with increasing ANC (>4000/μL), but with persistently normal s-GMIs and no other etiology for the infiltrates, a diagnosis of pulmonary inflammatory immune reconstitution syndrome (PIRIS)9 was made, and voriconazole continued. On day +24, he developed early respiratory failure, which rapidly responded to a 3-day course of 2 mg/kg of IV methylprednisolone per day. He was discharged in CR on oral voriconazole until resolution of IPA, with secondary voriconazole prophylaxis throughout consolidation. One year after diagnosis, he remained in sustained CR and free of IPA. This case illustrates several points relevant to IFDs in this patient population, including prevention (fluconazole prophylaxis), early diagnosis (serial s-GMI, cultures, chest CT findings, and confounding presentations such as PIRIS), and treatment (voriconazole), as well as the protective effects of remission status and secondary prophylaxis.
What has changed in the epidemiology of IFDs?
Much has changed in the epidemiology and management of IFDs since our 1995 review in Blood.10
Increasing mold infections
Invasive candidiasis (IC) was the most frequent IFD in patients with A-Leuk until the introduction of fluconazole,11 which led to a decrease in its incidence, a shift from susceptible (Candida albicans and C tropicalis) to more resistant species (C glabrata and C krusei),12 and an increase in invasive mold infections (IMIs), particularly invasive aspergillosis (IA), followed by fusariosis, mucormycosis, and others.13,14 Prophylaxis with the newly available mold-active triazoles—voriconazole and posaconazole—reduced the incidence of IA but has been associated with breakthrough IMIs with non-Aspergillus molds, particularly mucormycosis.15 In vitro resistance of Aspergillus species to triazoles is reported,16 although its clinical relevance remains unclear.
Geographic and seasonal variations
The epidemiology of IMI is subject to geographic and seasonal variations. For example, scedosporiosis is most frequently observed in Spain and Australia,17,18 whereas the incidence of fusariosis is highest in Houston, Texas,19 and Brazil.14 Seasonal variations in the incidence of aspergillosis and fusariosis were reported in Seattle, Washington,20 and Houston, Texas,19 respectively.
Waterborne IMIs
Patients with A-Leuk may acquire IMI from different sources, including air and water. Aspergillus species, Fusarium species, and the agents of mucormycosis were recovered from air, water, and water-related surfaces of hospitals,21 with demonstration of relatedness between patients’ and waterborne strains.22,23
What are the key risk factors for IFDs?
The risk for IFDs is higher among AML patients,13 except when ALL-intensified regimens are applied (Table 1). The highest rate is observed following salvage therapy for relapsed-refractory leukemia, although a significant proportion of newly diagnosed patients undergoing remission-induction develop IFDs, most commonly sinus, pulmonary aspergillosis (9% to 15%), or both.24-26 Despite long duration of severe neutropenia (DON; ANC <100/μL, ≥10-14 days), the rate of IA is lower after consolidation therapy (5.7%),27 reflecting the protective effect of CR status.25,26
Similarly, patients with ALL develop IFDs during remission induction26,28,29 but may remain at risk after neutrophil recovery because of cumulative treatment-related immunosuppression28 and corticosteroid-induced hyperglycemia.30
Because of the heterogeneity of A-Leuk, the risk for IFDs is highly variable and results from interactions between net state of immunosuppression, organ dysfunction, and exposure to opportunistic fungi.31
Immunosuppression is highest among patients with AML and high-risk myelodysplasia (MDS) because of their older age4,5 and adverse cytogenetics (hence, lower CR probability),4-6 neutropenia at diagnosis,5,26 and prolonged treatment-related DON.32 Cumulative corticosteroid doses25,33 and phagocytic dysfunction with antecedent MDS contribute to immunosuppression.34,35
Organ dysfunction increases IFD risk.4 For example, mucositis following mucotoxic regimens increases the risk for IC36 because Candida species are normal colonizers of gut mucosa,37 whereas preexisting lung pathology, including chronic obstructive pulmonary disease38 and smoking,39 predisposes patients to IA/IMIs following exposure to airborne conidia.25
Pretreatment risk assessment: leukemia genetics as markers for prolonged neutropenia and risk for IFDs
Because delayed diagnosis of IFDs is associated with higher morbidity and mortality, preventive measures should be taken as early as possible, preferably prior to antileukemic therapy.
Prolonged DON determines risk for IFD but because it is unknown prior to therapy, it is of limited value. Identifying pretreatment parameters that predict DON is therefore of paramount importance. Because the definition of CR requires an ANC >1000/μL, failure to enter remission is associated with longer DON and higher rates of severe infections, including IFDs32 and e-TRM.7 Hence, remission status that can be calculated prior to treatment predicts DON,42,43 with the caveat that unfit patients with favorable genetics may not survive long enough to achieve CR.43-45
Unfavorable prognostic factors for achieving CR and for e-TRM and toxic death include adverse cytogenetics and gene mutation profiles,46 elevated WBC counts,4 older age (arbitrary cutoff point of 65-75 years),44 and poor performance status.44
We use an online AML model (www.AML-score.org),42 which predicts probability of CR and toxic deaths among newly diagnosed patients (≥60 years old) eligible for intensive therapy.
The importance of pretreatment variables for risk for and outcome of IFDs is illustrated in a study evaluating risk factors for IA among 258 patients with A-Leuk.26 Median DON was 10 days longer among patients with IA than those without (31 vs 21 days, respectively), and DON was a risk factor for IA by univariate but not multivariate analysis. Only high-risk cytogenetics, neutropenia at diagnosis, and prior lung disease were independent predictors for IA. Strikingly, none of 33 patients with favorable cytogenetics developed IA vs 11 of 22 (50%) among those with adverse cytogenetics, and most patients who died of IA-related complications had refractory leukemia.26
The dominant role of remission status as risk and prognostic factor for IA is further highlighted in a study in which failure to enter CR and older age, but not neutropenia, were independent risk factors for IA following remission-induction for AML,32 and in another study in which progressive leukemia was more predictive of IA-attributable mortality than neutrophil recovery.47
Additional pretreatment risk factors (the most important shown in bold) are listed in Table 1.
Accordingly, a comprehensive assessment of pretreatment risk factors for infections in general, and IFDs in particular, should be part of routine evaluation in addition to leukemia diagnostics.
Day 15 posttreatment risk assessment
Another predictor of DON is day-15 blast count, as shown in a study of the impact of marrow evaluation for blast clearance: compared with patients with ≥5% day-15 blast count, those with ≤4% had shorter neutropenia (23 vs 33 days; P < .0001), lower rates of bloodstream infections (13.6% vs 23.2%; P = .003), and lower rates of aplastic death (1.8% vs 6.8%; P = .001).48
How do we translate risk factors into a risk-adapted and dynamic strategy to prevent IFDs?
Pretreatment assessment of risk for IFDs
Based on factors related to host, leukemia, and fungal exposure, we stratify patients into 3 risk categories for IFDs—high, intermediate, or low—and apply risk-adapted antifungal strategies accordingly.
A) High-risk group: patients with prior aspergillosis;49,50 those on salvage regimens for relapsed-refractory disease; and newly diagnosed patients undergoing remission-induction with any of the following risk factors: neutropenia at baseline,5,26 low CR probability,4-6 age ≥65 years,44 significant pulmonary dysfunction,38 and high e-TRM score4,44 (Table 1).
For these patients, we recommend a mold-active triazole (posaconazole or voriconazole) as primary or secondary prophylaxis, the latter in patients with prior IA or airway colonization with Aspergillus species.41,49
Antifungal prophylaxis for AML patients undergoing remission-induction therapy was evaluated in randomized controlled trials (RCTs). In one study, posaconazole recipients had significantly lower rates of IFDs (including IA) and a survival benefit compared with those randomized to fluconazole (or itraconazole).51 As a result, posaconazole is increasingly used as prophylaxis.52 Although voriconazole prophylaxis was not tested in a large RCT in the same setting, it is widely used as prophylaxis because of its efficacy in IA.53 Itraconazole is rarely considered for antifungal prophylaxis because of erratic bioavailability.
Problems associated with prophylaxis with mold-active triazoles include toxicity,54 treatment adherence,55 and variable bioavailability,56 in addition to increasing breakthrough IFDs15 and a significant decrease in the sensitivity of the s-GMI, the cornerstone of managing IA.57
Because of drug-drug interactions between antileukemic agents and mold-active triazoles, it is advisable to start the latter agents 24 hours after the last chemotherapy dose is infused.
In another RCT, prophylaxis with inhaled liposomal amphotericin B (L-AMB) reduced the rate of IPA, although 45% of patients discontinued prophylaxis for at least 1 week.58
B) Low-risk group: newly diagnosed young patients (≤45 years old) undergoing first remission-induction or consolidation therapy and without risk factors for IFDs. These patients may benefit from fluconazole prophylaxis, particularly if mucotoxicity is expected.36 Serial s-GMI testing is not recommended.
C) Intermediate-risk group: patients not meeting criteria for high- or low-risk groups. A diagnosis-driven preemptive antifungal therapy (DD-AFT) is best suited for these patients, using fluconazole prophylaxis59 with a switch to a mold-active agent after s-GMI seroconversion and compatible clinical and radiologic findings.60
The DD-AFT was evaluated in an RCT of 240 patients with A-Leuk and recipients of allogeneic hematopoietic cell transplantation randomized to DD-AFT (n = 118) or empiric therapy (n = 122).61 Blood samples for s-GMI and Aspergillus polymerase chain reaction were collected twice-weekly, and results only made available for DD-AFT recipients to allow mold-active therapy for seroconverters. Compared with empiric therapy, DD-AFT reduced the use of antifungal agents (15% vs 32%; P = .002) and increased the rate of IA diagnosis (15% vs 1%; P < .001), with 10 additional cases of IA diagnosed after retrospective analysis of s-GMI and polymerase chain reaction of empirically treated patients.
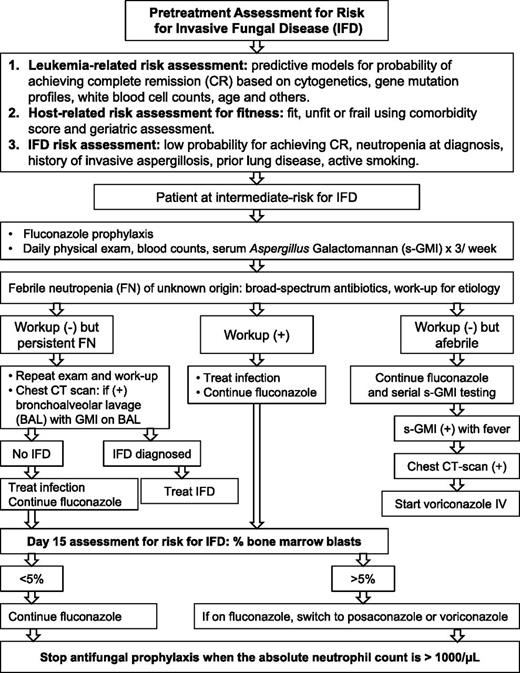
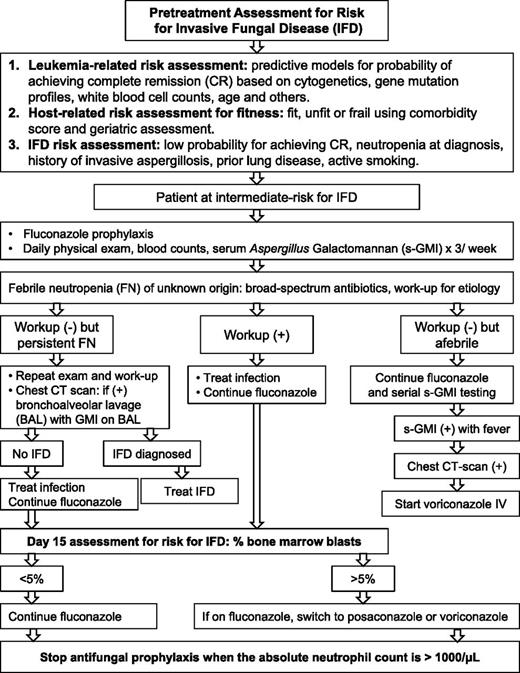
We recommend DD-AFT because it provides earlier and more frequent diagnosis of IA and prompt therapy for fewer patients, thereby reducing drug cost, toxicity, and drug-drug interactions. Moreover, the diagnostic certainty inherent to an Aspergillus-specific assay is informative for the type and timing of chemotherapy and the need for secondary prophylaxis.50 Figure 1 serves as an example of the DD-AFT management of patients at intermediate-risk for IFDs.
Risk-adapted antifungal strategy. Strategy based on pretreatment and day-15 posttreatment parameters for risk for IFDs in patients with AML undergoing remission-induction therapy. Example shown is for patients at intermediate risk for IFDs.
Risk-adapted antifungal strategy. Strategy based on pretreatment and day-15 posttreatment parameters for risk for IFDs in patients with AML undergoing remission-induction therapy. Example shown is for patients at intermediate risk for IFDs.
However, empiric therapy (ie, starting an antifungal agent after 4-7 days of antibiotic-refractory FN) is better suited at institutions at which s-GMIs are not resulted within 48 hours. The agent of choice depends on the antifungal prophylaxis received and may consist of an echinocandin, voriconazole, or L-AMB, the latter being the only option for recipients of mold-active prophylaxis because of infections with triazole-resistant molds, including mucormycosis.15
Dynamic day-15 posttreatment reassessment for risk for IFDs
Low- and intermediate-risk patients may be reclassified as high-risk for IFDs if their day-15 blast count is ≥5%.48 Prophylactic fluconazole is then replaced by a mold-active agent.
Other measures to prevent IFDs
The following 3 measures can reduce the morbidity and mortality of IFDs (Table 1):
1) Improving net state of immunosuppression by corticosteroid-dose reduction,25,33 intermediate-dose cytarabine consolidation for patients with favorable cytogenetics,43 and application of better-tolerated regimens; for example, arsenic trioxide with all-trans-retinoic acid for acute promyelocytic leukemia,64 tyrosine-kinase inhibitors plus corticosteroids for Philadelphia chromosome–positive ALL,65 first-line hypomethylating agents for elders with AML/MDS,66 and other promising regimens.67,68
A protective role for GM-CSF against infectious-related toxicities is strongly suggested in an RCT of older AML patients undergoing remission-induction therapy.69
G-CSF-elicited granulocyte transfusions may serve as a bridge in severely neutropenic patients, in whom neutrophil recovery is not expected within 3 to 4 days.70 Interferon γ, GM-CSF, or both may be useful in nonneutropenic patients, although solid evidence for these strategies is lacking.71
Lastly, unexpectedly prolonged DON requires evaluation to determine whether it is related to one or more concomitant viral infections, particularly cytomegalovirus, among patients treated for ALL.
2) Optimizing organ function, particularly pulmonary, by preventing respiratory viral infections, avoiding sick visitors,72 and smoking cessation, particularly among patients with chronic obstructive pulmonary disease38 (Table 1).
3) Decreasing exposure to opportunistic fungi by reducing primarily airborne conidia with high-efficiency particulate air filters in patients’ rooms73 and by taking special precautions during any building construction or demolition.74 Reducing secondarily airborne conidia from a water source75 may also be beneficial.
How do we secure an early diagnosis of IFD?
Common manifestations of IFDs during aplasia
The clinical manifestations of IFDs depend on the site of infection, the severity and dynamic nature of immunosuppression, and the infecting pathogen. Hematogenous dissemination with or without metastatic skin lesions is the usual presentation of yeast infections (eg, Candida species), whereas angioinvasion with pneumonia and tissue infarction is the hallmark of mold infections (eg, Aspergillus species). A mixed presentation of hematogenous dissemination and angioinvasion occurs with infections caused by molds with adventitious sporulation; that is, those capable of hematogenous dissemination by yeast-like spores (eg, Fusarium species).31
During aplasia, unexplained fever is the most common presentation of IFDs, followed by pneumonia with or without sinusitis, and less commonly skin/soft tissue or disseminated infection.
Monitoring patients during aplasia includes history and physical examination, daily blood counts and serum C-reactive protein (CRP), and s-GMI thrice-weekly. Serial CRPs are obtained because of the high negative predictive value (NPV) of normal CRP for severe infections,76 whereas elevated values can suggest an infectious etiology in patients with IFDs who may not develop fever.60 For FN, an infectious disease workup is performed and IV broad-spectrum antibiotics are started. Additional workup is obtained for antibiotic-refractory FN, including chest CT even in the absence of pulmonary findings.76 Patients with negative serum fungal biomarkers but with radiologic findings suggestive of IFD should undergo bronchoalveolar lavage (BAL), with testing of BAL fluids for fungal biomarkers. Table 2 shows additional clinical, laboratory, and imaging findings suggestive of IFDs.
Diagnostic tools
Blood cultures have moderate sensitivity for hematogenous infections with yeasts77 and molds with adventitious sporulation78 but have no sensitivity for IA.19 Cultures, stains, and histopathology with direct examination of involved sites can be diagnostic (eg, metastatic skin lesions of candidiasis, trichosporonosis,79 and fusariosis).19,78
Detecting s-GMI and BAL are particularly helpful in diagnosing IA.80,81 The test’s performance in neutropenic patients with A-Leuk is excellent,82 allowing early diagnosis and treatment days before the overt manifestations of IA.60 Early treatment is likely responsible for improved outcomes in IA.83 The kinetics of s-GMI are critical for monitoring response because they correlate with outcome,62,84,85 with rapid and solid response predicted when s-GMI normalizes within 1 week after seroconversion,63 and for distinguishing progressive aspergillosis from other infections or PIRIS.9 The test’s sensitivity, however, is reduced with mold-active prophylaxis.57 The occasional problem of false-positive values is minimized when testing is repeated before infusing broad-spectrum antibiotics. The test is also diagnostic in other IMIs such as fusariosis (sensitivity and specificity of 83% and 67%, respectively).78 We do not consider this result a false positive but a true positive for an IMI, which can be differentiated from IA by its distinguishing clinical and laboratory findings.19
Detecting circulating serum 1,3-β-D glucan (s-BDG) may be useful in diagnosing various IFDs, including candidiasis, aspergillosis, fusariosis, and others86,87 ; for example, s-BDG was positive in all 10 patients with fusariosis and preceded manifestations by 7 days.88 s-BDG may also be diagnostic for pneumocytosis, a concern in patients with ALL. However, false-positive s-BDG results are common due to several causes86,87 and limit the test’s predictive value, although persistently negative results have a high NPV for IFD.89 The combination of negative s-GMI89 and s-BDG90 practically excludes IFDs, except mucormycosis.82,90
Chest CT can suggest a diagnosis of IFD,82 although findings vary according to host immunity and may occur with other IMIs91 and other infectious and noninfectious conditions.92 A halo sign93 may suggest early IPA; however, earlier nonspecific findings indicate IPA when serial s-GMI values are elevated.60,83 Larger nodules (>1 cm) may develop with subsequent cavitation with or without air crescent sign after neutrophil recovery.94 A reversed halo sign may suggest mucormycosis.91 Abdominal imaging can detect splenic and hepatic nodules suggestive of chronic disseminated candidiasis,95 and positron emission tomography may be useful in staging extent and response of IFDs but is not recommended.96
How do we treat IFDs?
The same principles outlined in “Other measures to prevent IFDs” are also applicable to treatment. Optimizing antifungal therapy is also critical and encompasses selecting the optimal agent, ensuring adequate drug exposure, managing drug-drug interactions, applying objective parameters for outcome assessment, and providing adequate duration of therapy (DOT). Except for hematogenous candidiasis, secondary prophylaxis is required if additional chemotherapy is planned (Table 3).
Selecting the optimal antifungal agent: an individualized approach
We consider several factors when selecting the antifungal agent, including host, pathogen, drug properties, and infection site or sites (Tables 3 and 4). The indications, dosage schedules of antifungal agents, and other treatment measures are shown in Table 3.
We start IV therapy and switch to an oral agent after improvement, provided treatment adherence is good and gut function is intact (Table 4). We select the oral agent based on recent patient exposure to the same antifungal class, and the agent's expected activity against IFDs. For fungi exhibiting variable susceptibilities (eg, Fusarium species97 to voriconazole, and mucormycosis to posaconazole), we perform antifungal susceptibility testing and switch to these agents accordingly. For immune enhancement, we decrease corticosteroid doses and consider G-CSF and granulocyte transfusions if neutrophil recovery is not expected within 3 to 4 days, as discussed above.
Echinocandins are the drug of choice for hematogenous candidiasis, despite their limited activity against C parapsilosis.98 An alternative is fluconazole, L-AMB, and possibly amphotericin B lipid complex. We discourage the use of deoxycholate amphotericin B because of unacceptable toxicity. We do not routinely remove central venous catheters unless the port or tunnel is infected or if candidemia persists after 5 days of adequate treatment, suggesting an endovascular source and therefore requiring central venous catheter removal and significantly longer DOT99 (Table 3).
Chronic disseminated candidiasis is treated with the same agents used for hematogenous candidiasis. Oral corticosteroids can accelerate clinical improvement if symptoms persist despite antifungal therapy.100 Radiologic persistence of lesions is not, by itself, indicative of active infection.
Voriconazole is the drug of choice for aspergillosis,53 with L-AMB101 as an alternative. A benefit from combination therapy is suggested by the findings of a RCT in which a nonsignificant trend (P = .08) for the primary end point (death at 6 weeks) favored voriconazole plus anidulafungin vs voriconazole plus placebo.102 This limited benefit is likely related to selecting a non-Aspergillus-specific end point.
Worsening pulmonary infiltrates94 coinciding with neutrophil recovery likely represents PIRIS,9 which is distinguished from progressive aspergillosis by a normal s-GMI.63
The outcome of invasive fusariosis remains largely dependent on persistent neutropenia, corticosteroid-induced immunosuppression, or both.33 Treatment options are shown in Table 3, with little data to support any of these choices. We start L-AMB or voriconazole (Table 3) and add the second drug in the absence of clinical response. After improvement, we switch to oral voriconazole if the organism is susceptible.97
We treat mucormycosis with L-AMB, debridement of necrotic tissue, and correction of metabolic abnormalities.103 Upon response, we add posaconazole if the organism is susceptible and continue both agents for 1 week to ensure steady-state plasma posaconazole concentrations (PPCs) and then discontinue L-AMB.
Ensuring adequate drug exposure: any role for therapeutic drug monitoring?
The PPCs in patients with A-Leuk are significantly decreased in the presence of mucositis or diarrhea,56,104,105 poor food intake,105 and concomitant receipt of proton pump inhibitors56 or chemotherapeutic agents.105 Lower PPCs have been associated with increased IFDs.104 Compared with oral posaconazole solution, a once-daily tablet significantly improves bioavailability.106 Posaconazole is now available intravenously. Voriconazole is available orally and intravenously and its plasma concentrations vary according to genotype and drug interactions; eg, voriconazole plasma concentrations are significantly decreased with coadministration of glucocorticoids.107
The role of therapeutic drug monitoring for oral triazoles is unclear because the optimal concentrations remain to be defined and consistently correlated with clinical efficacy. We perform therapeutic drug monitoring when oral triazoles are given as prophylaxis for patients at high-risk for IFD and for the treatment of IFD, when concerns exist about gut function, triazole-related toxicity, or unexplained therapeutic failure.
Managing drug-drug interactions
Different interactions with triazoles must be considered,104 and consultation with a pharmacist is recommended.
1) Interactions in which triazoles affect other drugs,108 requiring monitoring for clinical toxicity and toxic-range blood levels (eg, all-trans-retinoic acid, etoposide, vincristine,109 ifosfamide, and cyclophosphamide).108
2) Interactions in which other agents such as phenytoin, rifampin, and others decrease or increase triazole exposure (eg, protease inhibitors causing subtherapeutic or supratherapeutic levels of the antifungal agent).
Objective assessment of treatment response
How long do we treat an IFD?
The DOT is dictated by resolution of the IFD and recovery from neutropenia (ANC >1000/μL), immunosuppression, or both. Evidence-based guidelines for DOT in immunosuppressed nonneutropenic patients are limited,110 and extrapolation from guidelines for HIV-positive patients is suggested.111 When CD4 cells are not measured, we use the absolute lymphocyte count (ALC) to gauge severity of immunosuppression and consider it moderate if ALC is persistently ≤1000/μL, and severe if ALC is persistently ≤300/μL. Ideally, antifungal agents are stopped when the IFD resolves and ALC is consistently ≥1000/μL, absent other causes for lymphocytosis or lymphopenia, such as infections.
When is it safe to start/resume antileukemic therapy in patients diagnosed with an IFD?
A diagnosis of IFD delays chemotherapy and may compromise outcome.49 Hence, careful evaluation of the activity of IFD prior to commencing chemotherapy is critical and relies on objective parameters including fever, s-GMI,63 s-BDG, and CRP. Persistence of some imaging abnormalities does not imply active infection.96 In patients with negative s-GMI, s-BDG, or both at diagnosis, we rely on clinical and radiologic findings and normal CRP values to exclude IFD.
If an IFD is diagnosed, antifungal treatment should be started immediately and chemotherapy delayed until the IFD is controlled, except in rare settings requiring urgent antileukemic intervention. Cytoreduction for elevated peripheral blast count can be achieved with oral hydroxyurea.
How do we manage refractory IFDs?
Failure to respond to antifungal therapy is not uncommon in patients with A-Leuk112 and is related to persistent profound myelosuppression, immunosuppression, or both. Measures to enhance immunity in “Other measures to prevent IFDs” can be considered. However, every attempt should be made to exclude other causes, including wrong diagnosis (eg, PIRIS),9 mixed fungal and nonfungal infections, suboptimal dose schedule of antifungal agents, and residual effects of IFDs such as persistent neurologic deficits after cerebral infarcts caused by angioinvasive molds. Although acquired drug resistance is rare, primary resistance of Aspergillus species to azoles is an emerging problem in some European countries.113
Conclusions and future directions
IFDs represent an important cause of treatment failure in adults with A-Leuk. Because of leukemia’s heterogeneity, the risk for IFDs is highly variable. We therefore apply a risk-adapted antifungal strategy with strong emphasis on pretreatment and posttreatment variables to allow earlier and more individualized interventions.
Based on pretreatment factors related to host, leukemia, treatment regimen, and fungal exposure, we stratify patients as high, intermediate, or low risk for IFDs and apply measures to prevent and monitor IFDs accordingly. A day-15 blast evaluation allows further risk stratification. Patients at high risk for IFDs who have unfavorable biology or are unfit for therapy can benefit from less-toxic anti-leukemic regimens.64-68
Novel targeted therapies that can achieve higher rates of sustained CR among patients with adverse genetics offer the best promise for reducing the burden of IFDs in these patients.
Authorship
M.N. and E.A. wrote and edited the manuscript.
Conflict-of-interest disclosure: M.N. has received honoraria from Merck, Pfizer, Gilead, and Astellas. E.A. has received honoaria from Astellas, Onix, and Millenium.
Correspondence: Elias Anaissie, Hoxworth Center, 3130 Highland Ave, 3rd Floor, Cincinnati, OH, 45219-2316; e-mail: elias114@aol.com or anaissieeliasj@gmail.com.