Key Points
Both mature KIR+ and immature KIR− NK cells exert antileukemic activity toward pediatric BCP-ALL in vivo.
In vivo treatment with low-dose 5-aza-cytidine enhances immature and mature NK-cell counts and promotes antitumor response.
Abstract
Therapeutic natural killer (NK)-cell–mediated alloreactivity toward acute myeloid leukemia has largely been attributed to mismatches between killer immunoglobulin-like receptors (KIRs) on NK cells and their ligands, HLA class I molecules, on target cells. While adult acute B-cell precursor leukemia (BCP-ALL) appears to be resistant to NK-cell–mediated lysis, recent data indicate that pediatric BCP-ALL might yet be a target of NK cells. In this study, we demonstrate in a donor-patient–specific NOD.Cg-PrkdcscidIL2rgtmWjl/Sz (NSG) xenotransplantation model that NK cells mediate considerable alloreactivity toward pediatric BCP-ALL in vivo. Notably, both adoptively transferred mature KIR+ NK cells and immature KIR− NK cells arising early posttransplantation in humanized NSG mice exerted substantial antileukemic activity. Low-dose and long-term treatment of humanized NSG mice with the DNA-demethylating agent 5-aza-cytidine distinctly enhanced the antitumor response, interestingly without inducing common inhibitory KIR expression but rather by promoting the differentiation of various NK-cell precursor subsets. Collectively, these data indicate that the future design of innovative therapy protocols should consider further exploitation of NK-cell–mediated immune responses for poor prognosis pediatric BCP-ALL patients.
Introduction
Natural killer (NK) cells belong to innate lymphoid immune cells that contribute to antitumor responses without prior sensitization. One important receptor family that controls NK-cell activity constitutes the human leukocyte antigen (HLA) class I-restricted killer immunoglobulin-like receptor (KIR) family that comprises both inhibitory and activating family members, which recognize allotypic variants of HLA class I alleles as KIR ligands (KIRL). In the setting of haplo-identical hematopoetic stem cell transplantation (HSCT), NK cells maturing in a HLA-disparate recipient will be shaped in the predominantly donor-type–like hematopoietic niche and will thus be donor-tolerant and recipient-“alloreactive,” at least during the first months after reconstitution.1-3 In this regard, the beneficial effects of promoting a certain degree of donor-recipient HLA-disparity was initially described by the Perugia group, who provided evidence that allogeneic HSCTs performed with grafts from KIR-KIRL mismatched donors promoted NK-cell alloreactivity, and enhanced survival rates in adult patients with acute myeloid leukemia (AML) but not with B-cell precursor leukemia (BCP-ALL).4,5 As a result of this apparent resistance of adult BCP-ALL to NK-cell–mediated lysis, the usefulness of NK-cell immune responses in treating BCP-ALL has been questioned and research in this field has largely been neglected. However, recent data indicate that the disease entity of pediatric BCP-ALL might yet be a target of “alloreactive” NK cells.1,2,6-8 Considering the various potential facets of NK-cell immune therapy, such as adoptive transfer of mature NK cells, co-transfer of mature NK cells during graft manipulation, emergence of immature NK cells early posttransplantation, and the currently existing limited number of clinical studies in BCP-ALL–bearing children, we sought to define conditions under which NK-cell–mediated alloreactivity toward pediatric BCP-ALL could be fully exploited.
Materials and methods
Mice
NOD.Cg-PrkdcscidIL2rgtmWjl/Sz (NSG) mice were purchased at The Jackson Laboratory and maintained under specified pathogen-free conditions in the research animal facility of the University of Tuebingen, Germany. All experimental animal procedures were conducted according to German federal and state regulations.
Induction of leukemia in NSG mice
Patient-specific leukemia was induced in NSG mice as described before.9 The study was approved by the local ethics committee and written informed consent was obtained from the parents, in accordance with the Declaration of Helsinki. Upon engraftment, mice were euthanized, and bone marrow (BM) or spleen specimens of leukemia-bearing mice were stored at −80°C for de novo generation of patient-specific leukemia at later time points.
NK-cell activation and expansion system (NKAES)
Peripheral blood mononuclear cells from HLA-typed donors (sequence-based typing with 4-digit resolution) (see supplemental Table 1 on the Blood Web site) were used with informed consent to expand NK cells in a medium containing 100 IU/mL IL2 (Novartis, Switzerland) using irradiated, membrane bound (mb)IL15-41BBL-expressing K562 cells, generously provided by D. Campana (University Children’s Hospital, Singapore) at a ratio of 1:1.5.10 To expand NK cells in a good manufacturing process-guided fashion, pooled human fresh frozen plasma was used instead of fetal calf serum. Expanded cells were harvested after 14 days and frozen in the presence of 10% dimethylsulfoxide at −80°C. Prior to experiments, NKAES cells were thawed and cultured overnight in the presence of 100 IU/mL IL2 for in vitro cytotoxicity assays or adoptive transfer experiments, and 200 IU/mL IL2 for functional NK-cell response staining or alloreactive NK-cell subset analysis.
Adoptive NK-cell transfer
Donor-patient pairs were selected that provided a mismatch or match in their KIR-KIRL repertoire (Table 1; supplemental Tables 1 and 2) and 1 × 106 blasts were IV injected into unirradiated NSG mice on day 0, followed by IV injection of 10 × 106 NKAES cells at the indicated time points. To monitor engraftment of leukemia, peripheral blood mononuclear cells of NSG mice were subjected to flow-cytometric analysis using formerly defined patient-specific leukemia surface markers.
Humanization of NSG mice
Humanization of NSG mice was performed as described before.11 Following approval by the local ethics committee, parents donated <5% of the hematopoietic stem cells (HSCs) for humanization of NSG mice. In selected experiments, HLA-typed HSCs were purchased from Key Biologics (Memphis, TN). To induce maturation of NK cells, humanized NSG (huNSG) mice were treated at 12 weeks posttransplantation with a complex of IL15/IL15Rα.12 In addition, huNSG mice were injected once on day 15 postinitiation of IL15 stimulation by intraperitoneal administration of 100 µg poly I:C (Sigma-Aldrich, Taufkirchen, Germany).13
Determination of in vivo cytotoxicity
Individual donor-patient pairs were selected that provided a mismatch or a match with respect to their KIR-KIRL repertoire. Mice were transplanted with HSCs of the respective donor and activated as described above at 12 to 20 weeks posttransplantation. To study NK-cell–mediated cytotoxicity, 3 × 106 blasts were IV injected into huNSG mice and the extent of blasts was determined at 20 hours postinjection in the BM using polychromatic (8 to 11) color flow cytometry. In some experiments, a mixture of 3 × 106 NSG-derived blasts (total 6 × 106 blasts) of 2 patients with varying KIR-KIRL repertoires were injected into huNSG mice and leukemia was quantified using the hierarchical gating strategy depicted in supplemental Figure 5A. In all experiments, the frequencies of patient-specific vital blasts were normalized to vital murine CD45+ cells.
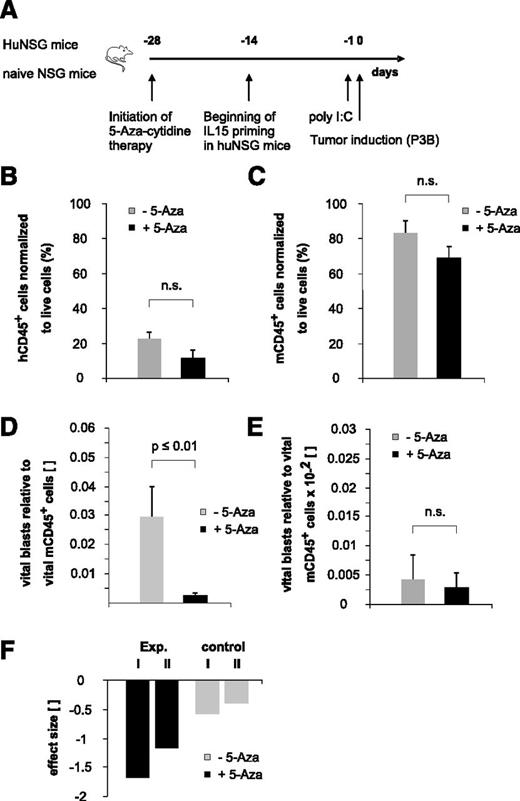
In vivo treatment of huNSG mice with 5-aza-cytidine
On day 39 posttransplantation, huNSG mice were randomly assigned to treatment or control group and therapy with 5-aza-cytidine (0.025 mg/mouse/dose intraperitoneally, twice a week for a total of 4 weeks) or phosphate-buffered saline as control was initiated. Four weeks later, 3 × 106 blasts were IV injected into 5-aza-cytidine or sham-treated animals. Some 20 hours later, mice were euthanized and subjected to analysis of NK-cell phenotype and for quantification of leukemic burden.
Functional NK-cell response staining
Sorted KIR+ and KIR− (for the respective sorting KIR mAb cocktail see supplemental information “Determination of in vitro cytotoxicity”) NKAES subsets were cocultured for 6 hours with NALM-16 or K562 cells (E:T ratio of 1:2) in the presence of CD107a-APC (H4A3) (BD Pharmingen). Subsequently, NK cells were stained with the indicated surface antibodies, permeabilized, and co-stained with the respective intracellular antibodies (perforin-PB [dG9] and TNF-bv605 [MAb11]) (BioLegend). For determination of IFN-γ, unsorted NKAES cells were cocultured with the respective target cells, subsequently stained with surface antibodies (including the above-mentioned anti-KIR antibody cocktail), and finally stained with IFN-γ–BUV395 (B27) antibody (BD Horizon). Percentages of the respective NK-cell subpopulation were then normalized to the baseline levels of NKAES cells cultured in control medium only. The specificity of the intracellular perforin staining was additionally verified by determining the perforin concentration in the coculture supernatants using the Diaclone Perforin ELISA Kit (Active Bioscience, Germany).
Alloreactive NK-cell subset analysis
Unsorted NKAES cells were cocultured with the respective target cells in the presence of CD107a-bv421 (H4A3) and subsequently stained with the following mAbs: KIR3DL1 (DX9; BD Pharmingen), KIR2DL2/L3/S2 (GL183; Beckman Coulter), KIR3DL1/S1 (Z27.3.7; Beckman Coulter), and KIR2DL1/S1/S4 (HP-3E4; BD Pharmingen). The selective combination of anti-KIR mAbs with different or identical fluorochromes hereby allowed the discrimination of KIR−, nonalloreactive or alloreactive KIR+ NK-cell subsets in the context of Bw4/C1 target cell recognition.
Statistics
Statistical advice was obtained from M. Eichner (Department of Clinical Epidemiology and Applied Biometry, University of Tuebingen, Germany) and M. Urschitz (Institute of Medical Statistics, University of Mainz, Germany). Mean values and SEM from experiments with 2 conditions were analyzed with the Student t test. The effect size Θ was calculated using the standardized mean difference between 2 populations: Θ = (μ1 to μ2) / σ, where μ1 and μ2 represent the mean values of the 2 study groups and σ represents the SEM of the total study population.
Methods concerning flow-cytometric analyses, determination of in vitro cytotoxicity, and KIR–quantitative polymerase chain reaction are referred to in supplemental “Materials and methods.”
Results
KIR-KIRL mismatch constellations promote the alloreactivity of cytokine-matured NK cells toward pediatric BCP-ALL in vitro and in vivo
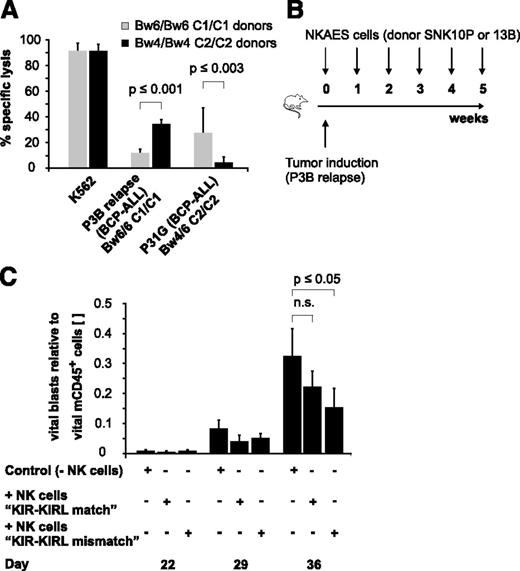
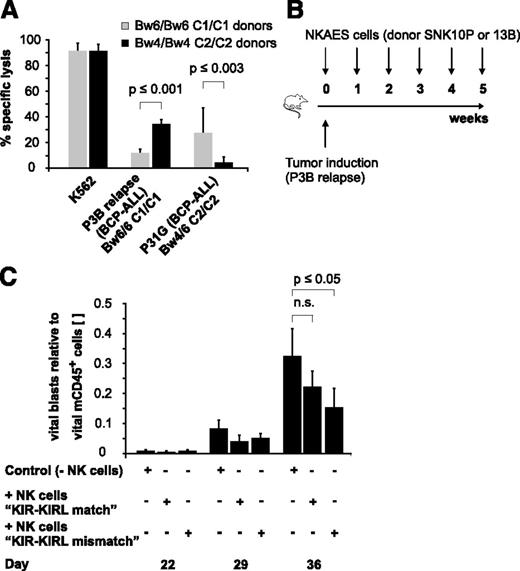
To characterize the alloreactivity of mature NK cells in various KIR-KIRL repertoire constellations, we applied a previously described good manufacturing process-guided NK-cell activation and expansion system (NKAES) and generated large numbers of cytokine-matured NK cells of donors with defined HLA class I genotypes (supplemental Table 1), KIR repertoires (supplemental Table 2), and NK-cell surface phenotypes (supplemental Figure 1A-B). We then chose a pediatric AML (Kasumi-1) and a BCP-ALL cell line (NALM-16), which express important NK-cell–receptor ligands to a comparable and significant extent (supplemental Figure 1C) to test whether KIR-KIRL mismatched NK cells can, in principle, target pediatric BCP-ALL. Indeed, KIR-KIRL mismatched NK cells exerted alloreactivity not only toward a pediatric AML but also toward a BCP-ALL cell line (supplemental Figure 2A). Comparative analysis of in vitro cytotoxicity assays performed with other pediatric BCP-ALL cell lines hereby demonstrated that this alloreactivity was clearly dependent on the extent of NKG2DL expression (supplemental Figure 2B-C). To further describe the relevance of KIR-KIRL interactions that might provide functional NK-cell competence, in addition to the immanent NKG2D-restricted activation, we performed cytotoxicity assays with donors who exhibited either a KIR-KIRL mismatch or a match toward two given BCP-ALL specimens (P3B relapse and P31G) (Table 1). In vitro cytotoxicity assays demonstrated that alloreactivity to BCP-ALL was better for those NK cells that were not subject to inhibition by self-HLA molecules (Figure 1A). In addition, injection of NKAES cells from a KIR-KIRL mismatched donor into P3B-engrafted NSG mice resulted in a higher reduction of tumor burden than transfer of the respective control NK cells (Figure 1B-C).
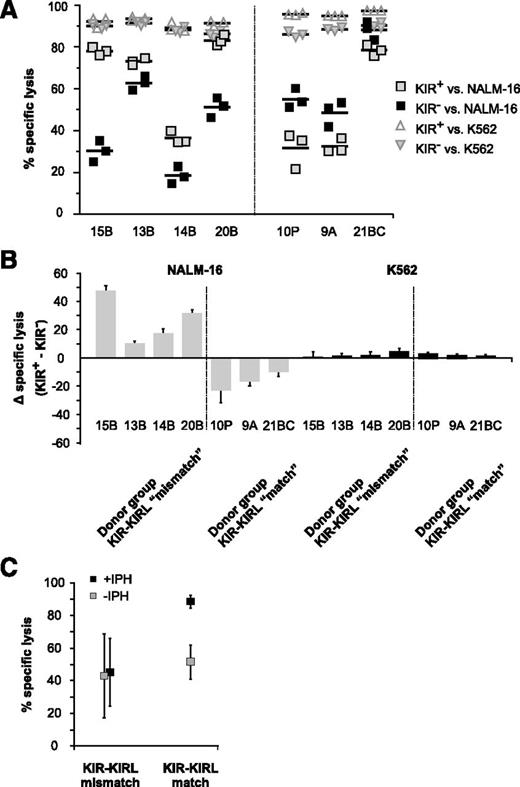
KIR-KIRL mismatch constellations promote the alloreactivity of cytokine-matured NK cells toward pediatric BCP-ALL in vitro and in vivo. (A) In vitro NK-cell alloreactivity toward BCP-ALL is donor-dependent. Shown is the specific lysis of KIR-KIRL mismatched or matched donors toward the two chosen BCP-ALL samples, P3B relapse and P31G (E:T ratio of 10:1). Cytotoxicity toward K562 cells is included as positive control. Data represent 2 independent experiments performed in triplicates. Donor/patient-specific KIR-KIRL repertoire constellations of the 6 donors SNK9A, SNK10P, SNK21BC, SNK13-15B are depicted in Table 1. (B-C) Donor selection influences the in vivo alloreactivity of NKAES cells toward pediatric BCP-ALL. (B) Experimental setup for Figure 1C. (C) Adoptively transferred NKAES cells of a KIR-KIRL mismatched donor (SNK13B) exert higher in vivo alloreactivity toward P3B than control NKAES cells of a KIR-KIRL matched donor (SNK10P). Data are representative of 1 experiment performed with 11 mice.
KIR-KIRL mismatch constellations promote the alloreactivity of cytokine-matured NK cells toward pediatric BCP-ALL in vitro and in vivo. (A) In vitro NK-cell alloreactivity toward BCP-ALL is donor-dependent. Shown is the specific lysis of KIR-KIRL mismatched or matched donors toward the two chosen BCP-ALL samples, P3B relapse and P31G (E:T ratio of 10:1). Cytotoxicity toward K562 cells is included as positive control. Data represent 2 independent experiments performed in triplicates. Donor/patient-specific KIR-KIRL repertoire constellations of the 6 donors SNK9A, SNK10P, SNK21BC, SNK13-15B are depicted in Table 1. (B-C) Donor selection influences the in vivo alloreactivity of NKAES cells toward pediatric BCP-ALL. (B) Experimental setup for Figure 1C. (C) Adoptively transferred NKAES cells of a KIR-KIRL mismatched donor (SNK13B) exert higher in vivo alloreactivity toward P3B than control NKAES cells of a KIR-KIRL matched donor (SNK10P). Data are representative of 1 experiment performed with 11 mice.
The KIR+ NK-cell subset of KIR-KIRL mismatched donors exerts higher cytotoxicity toward BCP-ALL than the corresponding KIR− subset
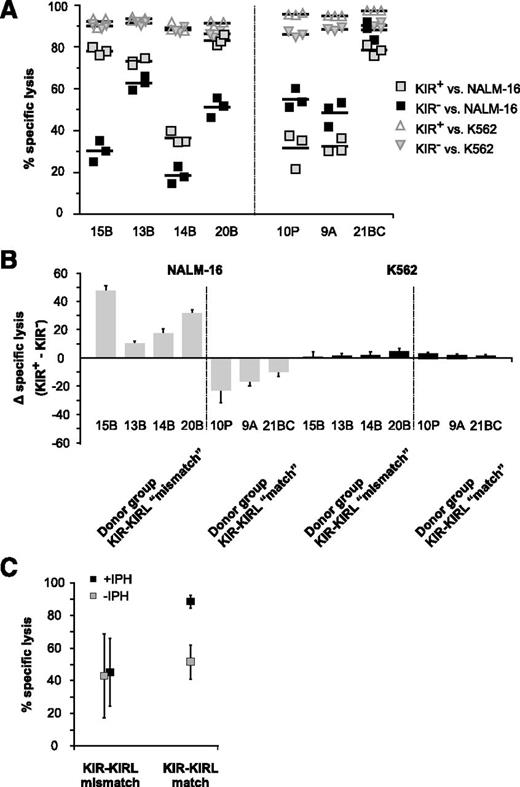
To further characterize the functional repertoire of NKAES cells with regard to potential KIR-KIRL mismatch constellations, we sorted KIR+ and KIR− NK-cell subsets. The KIR+ NK-cell subset of KIR-KIRL mismatched donors uniformly demonstrated higher levels of cytotoxicity when compared with the respective KIR− controls (Figure 2A), a phenomenon that became even more evident upon normalization (Figure 2B). In contrast, the KIR+ NK cells of matched donors invariably exhibited impaired cytotoxicity, indicating that functionality might have been compromised in response to inhibitory KIR-KIRL interactions. Cytotoxicity assays performed in the presence of the common inhibitory KIR2DL1 and 2DL2/3-blocking mAb IPH210214 revealed that the lytic activity of KIR+ NK cells of KIR-KIRL matched, HLA-C1 homozygous donors must have been controlled by inhibitory interactions of KIR2DL2 and 2DL3 receptors with their respective HLA-C1 group ligands expressed on NALM-16 cells (Figure 2C). In contrast, the KIR2DL2 and 2DL3+ NK-cell subset of our KIR-KIRL mismatched HLA-C2 homozygous donors did not respond to inhibitory KIR blockade.
The KIR+ NK-cell subset of KIR-KIRL mismatched donors exerts higher cytotoxicity toward pediatric BCP-ALL than the corresponding KIR− subset. (A) Sorted KIR+ and KIR− NK cells of the 7 donors characterized in Table 1 were cocultured with NALM-16 or K562 cells as a control to determine the extent of in vitro cytotoxicity (E:T ratio 5:1). (B) Standardization of the data depicted in Figure 2A. Shown is the specific lysis of the KIR+ NK-cell subset minus the one of the corresponding KIR− NK-cell subset. Data represent 6 independent experiments with 7 donors performed in triplicates. (C) Interactions of inhibitory KIRs with their cognate ligands determine the extent of NK-cell alloreactivity toward pediatric BCP-ALL. Alloreactivity of sorted KIR+ NKAES cells of the donors SNK14B, SNK15B, and SNK20B (KIR-KIRL mismatch), and SNK10P and SNK21BC (KIR-KIRL match) against NALM-16 was determined by in vitro cytotoxicity assays (E:T ratio 2:1) in the presence or absence of the common inhibitory KIR-blocking mAb, IPH2102. Data represent 5 independent experiments performed in triplicates.
The KIR+ NK-cell subset of KIR-KIRL mismatched donors exerts higher cytotoxicity toward pediatric BCP-ALL than the corresponding KIR− subset. (A) Sorted KIR+ and KIR− NK cells of the 7 donors characterized in Table 1 were cocultured with NALM-16 or K562 cells as a control to determine the extent of in vitro cytotoxicity (E:T ratio 5:1). (B) Standardization of the data depicted in Figure 2A. Shown is the specific lysis of the KIR+ NK-cell subset minus the one of the corresponding KIR− NK-cell subset. Data represent 6 independent experiments with 7 donors performed in triplicates. (C) Interactions of inhibitory KIRs with their cognate ligands determine the extent of NK-cell alloreactivity toward pediatric BCP-ALL. Alloreactivity of sorted KIR+ NKAES cells of the donors SNK14B, SNK15B, and SNK20B (KIR-KIRL mismatch), and SNK10P and SNK21BC (KIR-KIRL match) against NALM-16 was determined by in vitro cytotoxicity assays (E:T ratio 2:1) in the presence or absence of the common inhibitory KIR-blocking mAb, IPH2102. Data represent 5 independent experiments performed in triplicates.
The alloreactive KIR+ NK-cell subset of KIR-KIRL mismatched donors exhibits a superior ability for degranulation in response to pediatric BCP-ALL
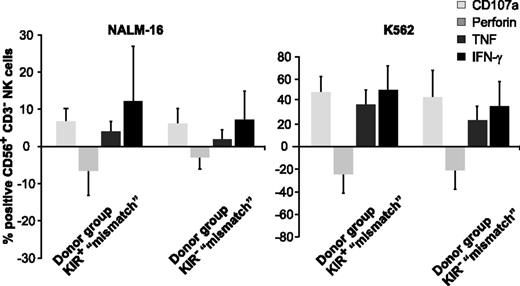
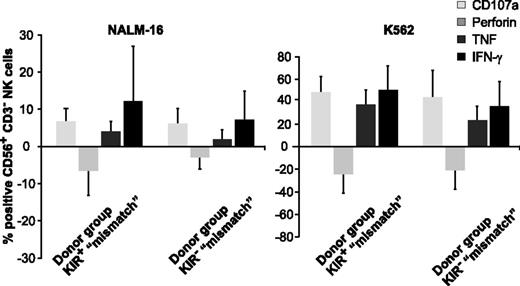
Facing this high cytotoxic activity of KIR+ NK cells toward KIRL-negative BCP-ALL target cells, one would expect significant differences in assays quantifying the ability for cytokine secretion or degranulation. Indeed, KIR-KIRL mismatch constellations boosted to some extent, the ability of KIR+ NK cells for both (Figure 3 and supplemental Figure 3), however, these data did not reach statistical significance. To further dissect the extent of antitumor responses of KIR+ NK cells, we analyzed the ability for degranulation in the alloreactive and nonalloreactive NK-cell subsets (Figure 4A) (for the respective NK-cell phenotype, see supplemental Figure 1B). Alloreactive KIR+ NK cells from mismatched donors revealed a superior capacity for degranulation in comparison with alloreactive KIR+ NK cells from matched donors (Figure 4B-D). This was first of all true for the KIR+NKG2A− NK-cell subset, but to some extent also for the cytokine-activated, and thus expanded the KIR+NKG2A+ subset. Interestingly, the KIR-KIRL mismatched donor SNK14B had very little alloreactive KIR+ NK cells (supplemental Figure 4), explaining the comparatively poor killing ability depicted in Figure 2A. In summary, we at this point, conclude that cytokine-matured NK cells of KIR-KIRL mismatched donors can exert a substantial cytotoxicity toward pediatric BCP-ALL cells, which is above all conferred by vivid degranulation of alloreactive KIR+ NK cells.
KIR-KIRL mismatch constellations boost the capacity for degranulation and cytokine synthesis of KIR+ NK cells toward pediatric BCP-ALL. Sorted KIR+ and KIR− NK cells of the donors SNK13-15B and SNK20B (donor group “mismatch”) were cocultured with NALM-16 or K562 cells as a control to determine the functional response in terms of degranulation and cytokine synthesis. Pooled data of the intracellular staining showing mean ± SEM. Given are the percentages of the respective CD107a+, perforin+, TNF+, and IFN-γ+ NK-cell subpopulation, normalized to the corresponding baseline levels of NKAES cells cultured in control medium only. Note that due to the genuinely higher response, the y-axis is differently scaled in K562 experiments. The negative bars indicate the decline in perforin levels that accompanies NK-cell degranulation.
KIR-KIRL mismatch constellations boost the capacity for degranulation and cytokine synthesis of KIR+ NK cells toward pediatric BCP-ALL. Sorted KIR+ and KIR− NK cells of the donors SNK13-15B and SNK20B (donor group “mismatch”) were cocultured with NALM-16 or K562 cells as a control to determine the functional response in terms of degranulation and cytokine synthesis. Pooled data of the intracellular staining showing mean ± SEM. Given are the percentages of the respective CD107a+, perforin+, TNF+, and IFN-γ+ NK-cell subpopulation, normalized to the corresponding baseline levels of NKAES cells cultured in control medium only. Note that due to the genuinely higher response, the y-axis is differently scaled in K562 experiments. The negative bars indicate the decline in perforin levels that accompanies NK-cell degranulation.
The alloreactive KIR+ NK-cell subset of KIR-KIRL mismatched donors exhibits a superior ability for degranulation in response to pediatric BCP-ALL. (A-D) NKAES cells of KIR-KIRL mismatched (SNK14B, SNK15B, and SNK20B) or matched donors (SNK9A, SNK10P, and SNK21BC), respectively, were cocultured with NALM-16 or K562 cells. Shown is the percentage of CD107a+ CD56+ population in various alloreactive or nonalloreactive NK-cell subsets. (A) Exemplified gating strategy for the identification of NK-cell subsets in donor SNK15B. In relation to NALM-16 cells (Bw4/C1), the potentially alloreactive NK-cell population (upper left square) is represented by the cells expressing KIR2DL1/S1/S4 and/or KIR3DS1 (y-axis) but not KIR2DL2/L3/S2 or KIR3DL1 (x-axis). (Note that the combined staining with a KIR3DL1/S1 [Z27.3.7] and a KIR3DL1 [DX9] antibody enables the identification of the KIR3DL1−S1+ cell population that belongs to the alloreactive subset). (B-C) Representative original histogram data obtained from a KIR-KIRL mismatched (SNK15B) and a matched donor (SNK10P). Data in (B) shows CD107a staining in NKAES-only cultures (gray, open) and NKAES cells cocultured with NALM-16 cells (black, open). Data in (C) shows the respective stainings in coculture experiments with K562 (black, open). The upper right histogram in Figure 4C delineates the respective isotype control (light gray, filled) that allows the subsequent marker position and identification of the CD107a+ subset in (B) and (C). In each histogram, the subtraction result ΔCD107a is given (% CD107a+ cells in NKAES-tumor cocultures minus % CD107a+ cells in NKAES-only cultures). Note that all experiments were performed with highly activated NKAES cells that exhibited a significant baseline CD107a expression. (D) Percentage of CD107a+ CD56+ cells in the indicated NK-cell subsets of KIR-KIRL mismatched (SNK14B, SNK15B, and SNK20B) or matched donors (SNK9A, SNK10P, and SNK21BC). Shown is the increase in CD107a expression of NALM-16 cocultured KIR+ NK-cell subsets normalized to the CD107a expression of the corresponding KIR− NK-cell subset. Filled black circle, KIR+ alloreactive subset in KIR-KIRL mismatched donors; open circle, KIR+ alloreactive subset in matched donors; light gray square, KIR+ nonalloreactive NK-cell subset. Data represents 1 experiment performed with the 6 indicated donors. n.s., not significant.
The alloreactive KIR+ NK-cell subset of KIR-KIRL mismatched donors exhibits a superior ability for degranulation in response to pediatric BCP-ALL. (A-D) NKAES cells of KIR-KIRL mismatched (SNK14B, SNK15B, and SNK20B) or matched donors (SNK9A, SNK10P, and SNK21BC), respectively, were cocultured with NALM-16 or K562 cells. Shown is the percentage of CD107a+ CD56+ population in various alloreactive or nonalloreactive NK-cell subsets. (A) Exemplified gating strategy for the identification of NK-cell subsets in donor SNK15B. In relation to NALM-16 cells (Bw4/C1), the potentially alloreactive NK-cell population (upper left square) is represented by the cells expressing KIR2DL1/S1/S4 and/or KIR3DS1 (y-axis) but not KIR2DL2/L3/S2 or KIR3DL1 (x-axis). (Note that the combined staining with a KIR3DL1/S1 [Z27.3.7] and a KIR3DL1 [DX9] antibody enables the identification of the KIR3DL1−S1+ cell population that belongs to the alloreactive subset). (B-C) Representative original histogram data obtained from a KIR-KIRL mismatched (SNK15B) and a matched donor (SNK10P). Data in (B) shows CD107a staining in NKAES-only cultures (gray, open) and NKAES cells cocultured with NALM-16 cells (black, open). Data in (C) shows the respective stainings in coculture experiments with K562 (black, open). The upper right histogram in Figure 4C delineates the respective isotype control (light gray, filled) that allows the subsequent marker position and identification of the CD107a+ subset in (B) and (C). In each histogram, the subtraction result ΔCD107a is given (% CD107a+ cells in NKAES-tumor cocultures minus % CD107a+ cells in NKAES-only cultures). Note that all experiments were performed with highly activated NKAES cells that exhibited a significant baseline CD107a expression. (D) Percentage of CD107a+ CD56+ cells in the indicated NK-cell subsets of KIR-KIRL mismatched (SNK14B, SNK15B, and SNK20B) or matched donors (SNK9A, SNK10P, and SNK21BC). Shown is the increase in CD107a expression of NALM-16 cocultured KIR+ NK-cell subsets normalized to the CD107a expression of the corresponding KIR− NK-cell subset. Filled black circle, KIR+ alloreactive subset in KIR-KIRL mismatched donors; open circle, KIR+ alloreactive subset in matched donors; light gray square, KIR+ nonalloreactive NK-cell subset. Data represents 1 experiment performed with the 6 indicated donors. n.s., not significant.
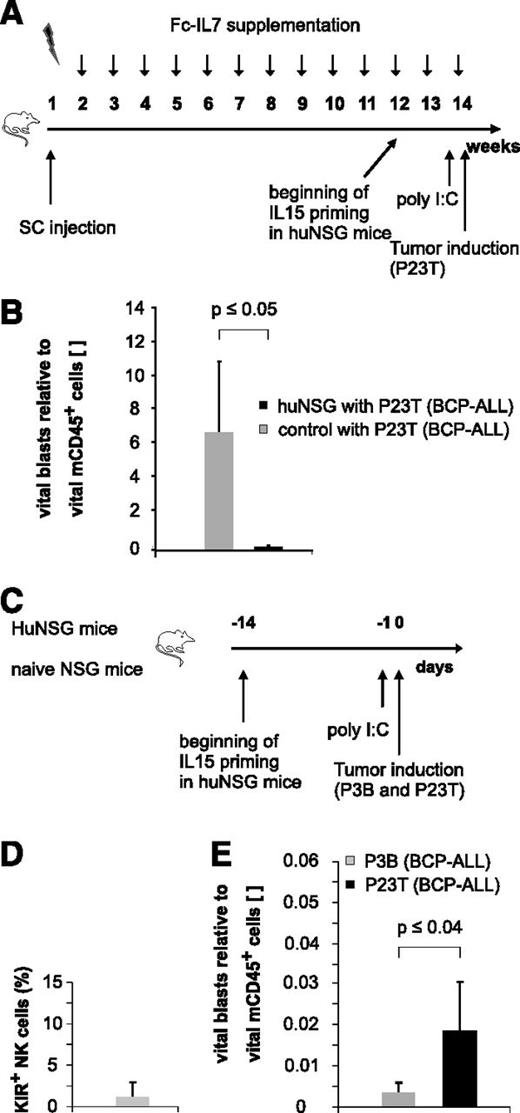
Immature, KIR− NK cells exert alloreactivity toward pediatric BCP-ALL
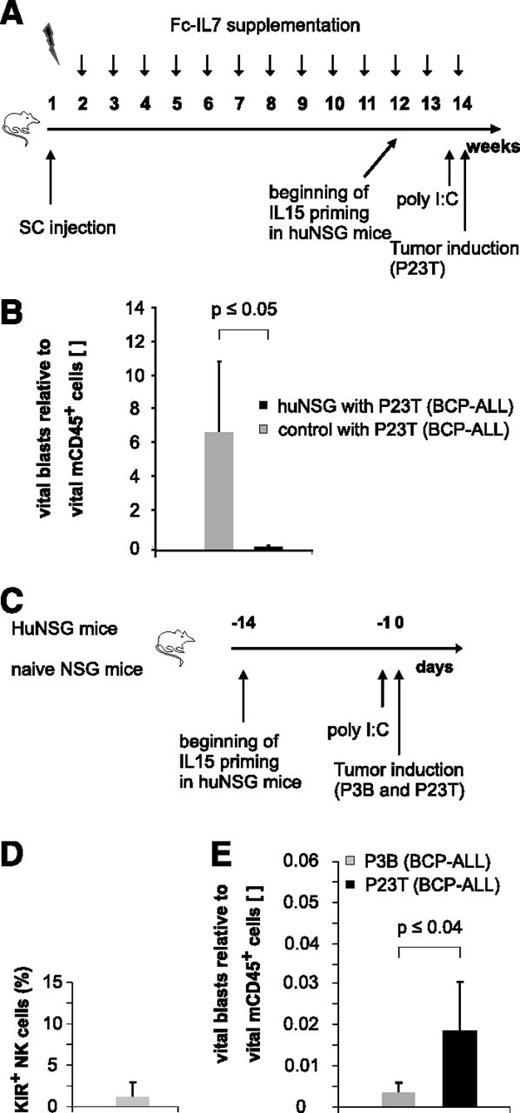
Since humans first reconstitute a large pool of immature KIR− NK cells early posttransplantation,2,15,16 we next asked whether these NK cells might also exert effector functions against pediatric BCP-ALL. Because KIR acquisition in NSG mice transplanted with human HSCs occurs only in a minor fraction of huNSG-derived NK cells (André and Münz, unpublished data), we chose this NSG xenotransplantation model as a surrogate model for the generation of “pseudo-mature lytic NK cells”17 arising in the early posttransplantation period in humans (Figure 5A). The injection of a primary BCP-ALL specimen (P23T) into huNSG mice prestimulated with IL15/IL15Rα and poly I:C, resulted in a substantial reduction of P23T tumor burden compared with control mice (Figure 5B). Since these results suggested that huNSG-derived NK cells may in principle be functionally active, we next injected a pooled sample of equal numbers of two primary BCP-ALL samples (P3B and P23T) with different KIRL repertoires into huNSG mice (Figure 5C). Applying the sequential gating strategy depicted in supplemental Figure 5A and verifying that the huNSG-derived NK cells were indeed to a large extent negative for common KIRs (Figure 5D), we found that the KIR-KIRL mismatched sample P3B was significantly better lysed than the matched sample P23T (Figure 5E). Of note, we deliberately euthanized huNSG mice 20 hours postinjection of leukemia to exclude for T-cell–mediated responses as a major cause of leukemia reduction. Since the alloreactivity toward P3B and P23T was reversible in experiments performed with a different HSC donor (supplemental Figure 5B), we concluded that the alloreactivity toward P3B depicted in Figure 5E was not a result of a P3B-intrinsic slower growth rate. Given that HLA class I, ICAM-1, NKG2D, and DNAM-1 ligands (supplemental Figure 1C) were expressed to a comparable extent by P3B and P23T, we assume that either KIR-KIRL interactions other than the one analyzed by us (KIR2DL1/S1/S4, KIR2DL2/S2/L3, and 3DL1) or modest differences (ie, in the expression of NKG2DL), must have contributed to this phenomenon.
Immature, KIR- NK cells exert alloreactivity toward pediatric BCP-ALL in vivo. (A) Experimental setup for humanization of NSG mice. (B) huNSG mice exhibit alloreactivity toward pediatric BCP-ALL in vivo. P23T was injected into huNSG or nonhumanized control mice, and leukemic burden was quantified 20 hours later in the BM. Given is the number of vital blasts normalized to vital murine CD45+ cells. Figure represents pooled data of 2 independent experiments obtained on a total of 7 huNSG and 3 control mice. (C) Experimental setup for Figure 5D-E. (D) KIR expression (KIR2DL1/S1/S4, KIR2DL2/L3/S2, and KIR3DL1) on BM-derived CD56+ NK cells of huNSG mice. (E) HuNSG-derived NK cells from SSC21D exert significantly higher in vivo alloreactivity against P3B than toward P23T. Given is the number of BM-residing vital blasts normalized to vital murine CD45+. Data are representative of 2 independent experiments with a total of 4 huNSG mice.
Immature, KIR- NK cells exert alloreactivity toward pediatric BCP-ALL in vivo. (A) Experimental setup for humanization of NSG mice. (B) huNSG mice exhibit alloreactivity toward pediatric BCP-ALL in vivo. P23T was injected into huNSG or nonhumanized control mice, and leukemic burden was quantified 20 hours later in the BM. Given is the number of vital blasts normalized to vital murine CD45+ cells. Figure represents pooled data of 2 independent experiments obtained on a total of 7 huNSG and 3 control mice. (C) Experimental setup for Figure 5D-E. (D) KIR expression (KIR2DL1/S1/S4, KIR2DL2/L3/S2, and KIR3DL1) on BM-derived CD56+ NK cells of huNSG mice. (E) HuNSG-derived NK cells from SSC21D exert significantly higher in vivo alloreactivity against P3B than toward P23T. Given is the number of BM-residing vital blasts normalized to vital murine CD45+. Data are representative of 2 independent experiments with a total of 4 huNSG mice.
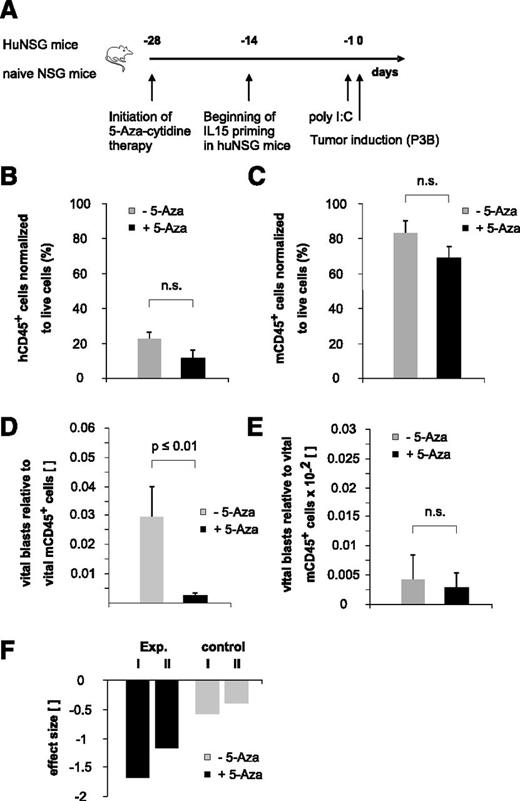
“Bridging” therapy with 5-aza-cytidine supports NK-cell alloreactivity toward pediatric BCP-ALL in the early posttransplantation period
To elaborate the full potential of the immature NK-cell pool arising early posttransplantation, we recapitulated existing in vitro data indicating that 5-aza-cytidine modulates NK-cell function by inducing KIR promoter activity18 and expression19 in our xenotransplantation model in the context of pediatric BCP-ALL disease. Because the therapeutic efficacy of DNA methyltransferase inhibitors may reach from the induction of DNA-demethylating effects at low doses to the induction of direct cytotoxic effects at higher doses, we decided to apply a low-dose treatment regimen for an extended period to potentially enable long-term epigenetic modulation of NK cells (Figure 6A). This treatment regimen resulted in a statistically not significant reduction of both human and murine CD45+ cells, reflecting to some extent a drug-induced BM cytotoxicity (Figure 6B-C). Interestingly, contrary to previously published in vitro data reporting a 5-aza-cytidine–induced functional inhibition of mature NK cells,20 we observed a clearly reduced BCP-ALL burden in 5-aza-cytidine–treated huNSG mice (Figure 6D). Of note, we had stopped the 5-aza-cytidine therapy 3 days before the injection of leukemia and the half-life of the drug is described to be less than 4 hours.21 Therefore, together with our data obtained in nonhumanized controls (Figure 6E,F), we can largely exclude a drug-induced, direct cytotoxic effect that might have reduced the tumor load.
“Bridging” therapy with 5-aza-cytidine supports NK-cell alloreactivity toward pediatric BCP-ALL in the early posttransplantation period. (A) Experimental setup. (B-C) Low-dose and long-term 5-aza-cytidine treatment does not exert statistically relevant BM cytotoxicity. Given is the number of human (B) or murine (C) CD45+ cells normalized to total live cells. Data depicted in Figure 6B were obtained in 5-aza-cytidine–treated huNSG mice, and data depicted in Figure 6C were obtained in 5-aza-cytidine–treated nonhumanized control mice. (D) Treatment with 5-aza-cytidine significantly reduces BCP-ALL tumor load in huNSG mice. (E) Low-dose 5-aza-cytidine treatment regimen does not exert relevant direct cytotoxic effects on pediatric BCP-ALL. Given is the number of vital blasts in the BM normalized to murine CD45+ cells in nonhumanized control NSG mice. (F) Calculated effect size of in vivo 5-aza-cytidine treatment on pediatric BCP-ALL burden. “Exp. I and II” denote the 2 different experiments in huNSG mice; “control I and II” denote the effect in the respective control groups. Data are representative of 2 independent experiments with a total of 11 huNSG mice and 14 control NSG mice. n.s., not significant.
“Bridging” therapy with 5-aza-cytidine supports NK-cell alloreactivity toward pediatric BCP-ALL in the early posttransplantation period. (A) Experimental setup. (B-C) Low-dose and long-term 5-aza-cytidine treatment does not exert statistically relevant BM cytotoxicity. Given is the number of human (B) or murine (C) CD45+ cells normalized to total live cells. Data depicted in Figure 6B were obtained in 5-aza-cytidine–treated huNSG mice, and data depicted in Figure 6C were obtained in 5-aza-cytidine–treated nonhumanized control mice. (D) Treatment with 5-aza-cytidine significantly reduces BCP-ALL tumor load in huNSG mice. (E) Low-dose 5-aza-cytidine treatment regimen does not exert relevant direct cytotoxic effects on pediatric BCP-ALL. Given is the number of vital blasts in the BM normalized to murine CD45+ cells in nonhumanized control NSG mice. (F) Calculated effect size of in vivo 5-aza-cytidine treatment on pediatric BCP-ALL burden. “Exp. I and II” denote the 2 different experiments in huNSG mice; “control I and II” denote the effect in the respective control groups. Data are representative of 2 independent experiments with a total of 11 huNSG mice and 14 control NSG mice. n.s., not significant.
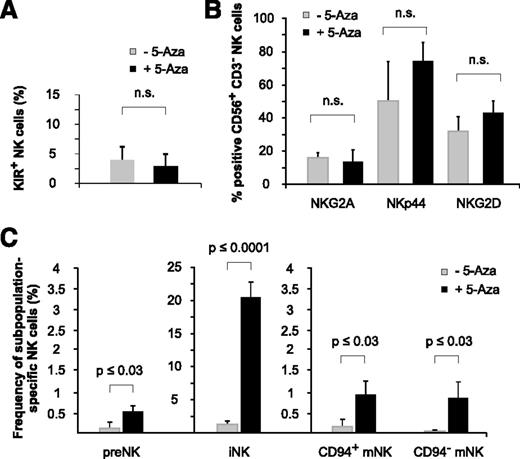
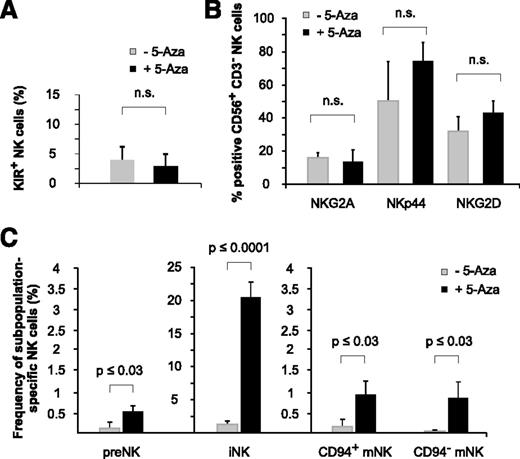
Low-dose and long-term exposure to 5-aza-cytidine promotes NK-cell ontogeny
NK-cell receptor analysis of huNSG mice revealed that 5-aza-cytidine did not significantly modulate the expression of selected NK-cell receptors (KIRs, NKG2A, NKp44, and NKG2D) at the low doses applied (Figure 7A-B). However, the analysis of NK-cell subsets interestingly showed that 5-aza-cytidine–treated huNSG mice had clearly higher numbers of both immature (CD34+CD117+ and CD34−CD117lowCD94−) NK-cell precursors and mature (CD34−CD117−CD94+NKp46+ and CD34−CD117−CD94−NKp46+) NK-cell subsets22 (Figure 7C), indicating that 5-aza-cytidine might have facilitated NK-cell ontogeny itself.
Low-dose and long-term exposure to 5-aza-cytidine promotes NK-cell ontogeny. (A-B) 5-Aza-cytidine does not significantly alter the expression of KIR2DL1/S1/S4, KIR2DL2/L3/S2, and KIR3DL1 (A), or other inhibitory or activating NK-cell receptors (B), on BM-derived NK cells of huNSG mice. (C) 5-Aza-cytidine induces the expression of BM-residing NK-cell precursors. Frequencies of the indicated NK-cell subpopulations in the BM of 5-aza-cytidine–treated or control huNSG mice (as defined by Freud22 ): preNK (CD34+CD117+), iNK (CD34−CD117lowCD94−), CD94+ mNK cells (CD34−CD117−CD94+NKp46+), and CD94− mNK cells (CD34−CD117−CD94−NKp46+). Data represent 2 independent experiments with a total of 11 huNSG mice and 14 control NSG mice. n.s., not significant.
Low-dose and long-term exposure to 5-aza-cytidine promotes NK-cell ontogeny. (A-B) 5-Aza-cytidine does not significantly alter the expression of KIR2DL1/S1/S4, KIR2DL2/L3/S2, and KIR3DL1 (A), or other inhibitory or activating NK-cell receptors (B), on BM-derived NK cells of huNSG mice. (C) 5-Aza-cytidine induces the expression of BM-residing NK-cell precursors. Frequencies of the indicated NK-cell subpopulations in the BM of 5-aza-cytidine–treated or control huNSG mice (as defined by Freud22 ): preNK (CD34+CD117+), iNK (CD34−CD117lowCD94−), CD94+ mNK cells (CD34−CD117−CD94+NKp46+), and CD94− mNK cells (CD34−CD117−CD94−NKp46+). Data represent 2 independent experiments with a total of 11 huNSG mice and 14 control NSG mice. n.s., not significant.
Discussion
Given the disparity of adult and pediatric acute lymphoid leukemia we in this study sought to investigate NK-cell–based immune responses to the tumor entity, pediatric BCP-ALL. Our data revealed that not only mature KIR+ but interestingly also immature KIR− NK cells controlled pediatric BCP-ALL in vivo, and indicate that future protocols should consider further exploitation of NK-cell–mediated immune responses for poor prognosis pediatric BCP-ALL patients.
The difference in the susceptibility of adult and childhood BCP-ALL to NK-cell–mediated lysis has been mainly ascribed to differing expression of cell adhesion molecules of the β1 and β2 integrin and the immunoglobulin superfamily, respectively, that essentially results in a reduced NK-cell target conjugate formation and activation in the case of adult BCP-ALL.4,23 In addition, the surface density of HLA class I ligands is greater and the expression of ligands to major activating NK-cell receptors, such as NKG2D, is lower in pediatric BCP-ALL as compared with AML.24 In line with this, our group earlier demonstrated that the in vitro cytolytic activity of NK cells against BCP-ALL in part correlated with the extent of major histocompatibility complex expression on the respective specimen.6,7
Per our previous work, which demonstrated that the injection of patient-specific leukemia into NSG mice resulted in the constitution of a model that reflected individual leukemogenecity,9 we analyzed the leukemic burden in BCP-ALL–bearing mice upon adoptive NK-cell transfer and demonstrated that KIR-KIRL mismatched NK cells targeted, but did not eliminate, pediatric BCP-ALL. Mechanistically, the interaction of NK cells with BCP-ALL was accompanied by a heightened functionality of alloreactive KIR+ NK cells of KIR-KIRL mismatched donors, particularly in terms of their ability for degranulation. As the presence of the inhibitory KIR-blocking mAb IPH2102 distinctly enhanced the cytotoxicity of matched KIR+ NK cells, we conclude that the KIR-KIRL axis indeed contributes to the control of NK-cell alloreactivity, and hypothesize that this antibody might provide clinically significant effects in selected young BCP-ALL patients. The absence of effectiveness of KIR blockade in our KIR-KIRL mismatched C2/C2 donors is difficult to explain as inhibitory signaling alone should be functional even in the KIR2DL2/L3+ NK cells of these donors. However, the KIR2DL2/L3+ NK-cell subset that lacked the coexpression of other inhibitory KIRs was comparatively large in our donors, and displayed, despite the presence of NKG2A, a low functional ability in terms of response to K562 (data not shown). Thus, we at this point assume that these two findings together might explain the decreased responsiveness to IPH2102 in our C2/C2 mismatched donors.
Irrespective of the individual KIR-KIRL constellation, the KIR- NK-cell subset interestingly enough displayed substantial functionality. In line with the previously published observation that the attenuated function of KIR− NK cells can be overcome by exposure to cytokines,25 we attribute this observation to the cytokine-rich expansion protocol.
The so far published data on adoptive NK-cell transfer in children is restricted to feasibility studies performed mostly in AML patients.26 Our group recently demonstrated that the infusion of IL15-stimulated CD3−/CD19− depleted HSC grafts (containing high numbers of NK cells) is safe, even in the haplo-identical setting.8 Given that NK cells can persist and expand in HLA-mismatched hosts,27 and that the transfer of mature NK cells does obviously not promote graft-versus-host disease,8,26 the data depicted in this study support the concept that the adoptive transfer of KIR+ NK cells of KIR-KIRL mismatched donors might indeed play a role in the treatment of relapsing BCP-ALL disease, not alone, but as an adjunct to haplo-identical HSCT.
To date, 2 clinical studies have evaluated the feasibility of KIR-KIRL mismatched haplo-identical HSCTs in children with BCP-ALL. Our group demonstrated that the graft-versus-leukemia (GVL) effect best correlated with the number of KIR-KIRL mismatches.1 The second study was performed by the Moretta group with the aim of better defining the specificity and clonal distribution of “alloreactive” NK cells.2 Collectively, these 2 preliminary studies show that the incorporation of theoretical assumptions on KIR-KIRL interactions in haplo-identical HSCTs of children with BCP-ALL is feasible, that such a mode of donor selection does not heighten the risk of graft-versus-host disease, and that the analysis of receptor-ligand constellations may have prognostic implications.
In this study, we systematically demonstrate in huNSG mice that emerging KIR− NK cells exert a considerable cytotoxicity toward BCP-ALL, thus extending earlier data that a substantial number of phenotypically healthy appearing NK cells exist in mice and men, that obviously lack the expression of any inhibitory receptor for “self”-HLA class I28,29 but nevertheless display a certain degree of functionality. In line with previously published data that “unlicensed” NK cells may exert a profound immune response in the context of murine cytomegalovirus infection,30 our data indicate that the antitumor properties of immature KIR− NK cells are substantial and might just as well have been previously underestimated. Since the KIR expression in huNSG mice was negligible despite the administration of IL15/IL15Rα complex, we at this point, do not ascribe the occurring NK-cell activation to the lack of KIR-KIRL–mediated inhibition but rather assume that receptor-ligand interactions other than the one analyzed by us must have contributed to this phenomenon.
Using a humanized xenotransplantation model to analyze GVL potency after haplo-identical HSCT is undoubtedly technically challenging, and has to our knowledge, so far not been attempted. The application of minimal residual disease (MRD) analysis based on multi (8 to 11) parameter flow cytometry enabled the distinction of immunophenotypic features of one (or two) patient-specific malignantly transformed B-cell specimen from early-arising donor-type–like B-cell–lineage precursors. This approach has allowed us to characterize GVL effects in a dynamic and largely functional hematopoietic system that incorporates complex effector-target cell interactions in the presence of “human-like” bystander cells and supportive cytokines.
As recent data indicate that DNA-demethylating agents may not only affect the growth of malignant cells but might also modulate inhibitory KIR expression on NK cells, we tested 5-aza-cytidine in our huNSG model. Interestingly, we observed a significantly reduced BCP-ALL burden in huNSG mice that could not be solely attributed to drug-induced, direct cytotoxic effects. This observation was in contrast to previously published in vitro data reporting a 5-aza-cytidine–induced functional inhibition of NK cells20,31 ; however, careful analysis of these data revealed that the knowledge of 5-aza-cytidine–mediated immune-modulation of NK cells is basically restricted to studies of mature NK cells of healthy volunteer donors. Analysis of BM-residing NK-cell subsets in huNSG mice demonstrated that 5-aza-cytidine–treated mice harbored distinctly higher numbers of both immature and mature NK-cell subsets. So far, it has been speculated that beneficial antitumor effects of 5-aza-cytidine should probably be attributed to the induction of HLA class I expression and cancer testis antigens on malignant or premalignant cell clones.32 In line with this tumor-focused concept, 5-aza-cytidine was used to “bridge” the early posttransplantation period in high-risk AML and chronic myelomonocytic leukemia patients.33,34 However, based on our results, we propose that 5-aza-cytidine therapy might induce a remodeling in developing NK-cell precursors, that presumably together with an altered dendritic cell functionality and a fine-tuned NK-cell–promoting cytokine milieu,35 leads to increased NK-cell numbers and improved functionality.
In line with the observation that 5-aza-cytidine may promote the differentiation of malignantly transformed cells by inducing the re-expression of epigenetically downregulated PU.136 and considering the crucial role that PU.1 plays in the regulation of NK-cell differentiation and homeostasis,37 we assume that the re-expression of PU.1 might have promoted NK-cell differentiation in our model. In addition, it has been shown for low (but not high dose) 5-aza-cytidine therapy that this may increase the number of cells in the S phase, and may thus promote cell-cycle progression.38 Recalling data that 5-aza-cytidine therapy does not evoke a broad and unspecific passive demethylation of the genome but rather induces a highly specific nonrandom demethylation pattern,38 we at this point hypothesize that low-dose 5-aza-cytidine might have selectively reshaped factors important for NK-cell transcription and cell-cycle control.
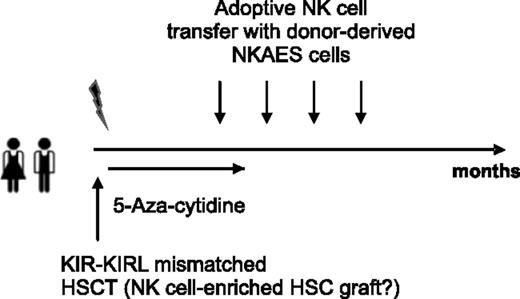
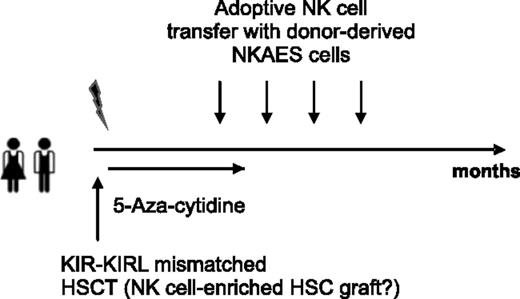
In conclusion, we provide in this study substantial evidence that both KIR+ and immature KIR− NK cells may exert clinically relevant GVL effects toward pediatric BCP-ALL. Our data therefore support the rationale for adoptive NK-cell transfer of ex vivo activated mature donor-specific NK cells that would chaperone the early, NK-cell–deficient phase of ideally KIR-KIRL mismatched haplo-identical HSCTs in children with relapsing BCP-ALL (Figure 8). The application of a low dose 5-aza-cytidine “bridging” therapy, potentially under supportive cytokine administration, might additionally promote NK-cell differentiation and functionality, and would thus constitute an optional treatment strategy for those patients lacking a suitable KIR-KIRL mismatched donor.
Chaperoning the NK-cell–deficient phase of haplo-identical HSC transplantation. Delineation of a hypothetical study design in relapsing pediatric BCP-ALL patients ideally exploiting NK-cell–mediated antitumor responses.
Chaperoning the NK-cell–deficient phase of haplo-identical HSC transplantation. Delineation of a hypothetical study design in relapsing pediatric BCP-ALL patients ideally exploiting NK-cell–mediated antitumor responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the children for donating BM samples at the time of diagnosis, their parents for donating HSCs for the humanization of NSG mice, and the healthy volunteers for repetitively donating large blood volumes for NK-cell isolation. We thank D. Wernet (Center for Clinical Transfusion Medicine, University of Tuebingen, Germany) for providing buffy coat preparations of healthy volunteers, B. Goecke and K. Stauss for excellent technical assistance, A. Steinle (University of Frankfurt, Germany) for generously providing anti-NKG2DL antibodies, W. Strittmatter (Merck KGaA, Darmstadt, Germany) for providing the human Fc IL-7 construct, and Bristol-Myers Squibb (Princeton, NJ) for donating the common inhibitory KIR-blocking mAb, IPH2102. The authors also thank M. Eichner (University of Tuebingen, Germany) and M. Urschitz (University of Mainz, Germany) for statistical advice, and M. Uhrberg (University Clinic of Duesseldorf, Germany) for the helpful discussions. The authors acknowledge the gift of the K562-mbIL15-41BBL cell line by D. Campana (National University of Singapore, Singapore) and thank C. Mueller (University of Tuebingen, Germany) for high resolution HLA class I typing of HSC and healthy NK-cell donors or patients, respectively.
This study was supported by grants from the Deutsche Forschungsgemeinschaft (KFO 183) (M.C.A., P.L., R.H., U.F.H., and W.H.), the Deutsche José Carreras Leukämie-Stiftung (12/03), the Faculty of Medicine of Tuebingen (E.05.00301.1), the Else Kröner-Fresenius-Stiftung (2012_A296) (M.C.A.), the Stiftung für krebskranke Kinder Tübingen e.V., and the Stefan-Morsch-Stiftung (R.H.).
Authorship
Contribution: A.K. and J.W. designed and performed experiments, interpreted the data, and critically revised the manuscript; K.E.W. designed the patient-specific MRD analysis, and contributed to performing and interpreting the MRD data obtained in mice; K.E.W. and H.J.B. advised and helped with cell sorting; M.E. provided patient samples; L.O. and M.M. established the KIR-quantitative polymerase chain reaction; P.L. obtained written informed consent of parents and patients; W.H. and R.H. interpreted the data; U.F.H. and C.M. interpreted the data and critically revised the manuscript; and M.C.A. conceptualized the work, designed experiments, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: M.C.A. and A.K. have filed a provisionary patent application on the use of IPH2102 in pediatric BCP-ALL. The remaining authors declare no competing financial interests.
Correspondence: Maya Caroline André, University Children’s Hospital, Department of Pediatric Hematology and Oncology, Hoppe-Seyler-Str.1, D-72076 Tuebingen, Germany; e-mail: maya.andre@med.uni-tuebingen.de.

![Figure 4. The alloreactive KIR+ NK-cell subset of KIR-KIRL mismatched donors exhibits a superior ability for degranulation in response to pediatric BCP-ALL. (A-D) NKAES cells of KIR-KIRL mismatched (SNK14B, SNK15B, and SNK20B) or matched donors (SNK9A, SNK10P, and SNK21BC), respectively, were cocultured with NALM-16 or K562 cells. Shown is the percentage of CD107a+ CD56+ population in various alloreactive or nonalloreactive NK-cell subsets. (A) Exemplified gating strategy for the identification of NK-cell subsets in donor SNK15B. In relation to NALM-16 cells (Bw4/C1), the potentially alloreactive NK-cell population (upper left square) is represented by the cells expressing KIR2DL1/S1/S4 and/or KIR3DS1 (y-axis) but not KIR2DL2/L3/S2 or KIR3DL1 (x-axis). (Note that the combined staining with a KIR3DL1/S1 [Z27.3.7] and a KIR3DL1 [DX9] antibody enables the identification of the KIR3DL1−S1+ cell population that belongs to the alloreactive subset). (B-C) Representative original histogram data obtained from a KIR-KIRL mismatched (SNK15B) and a matched donor (SNK10P). Data in (B) shows CD107a staining in NKAES-only cultures (gray, open) and NKAES cells cocultured with NALM-16 cells (black, open). Data in (C) shows the respective stainings in coculture experiments with K562 (black, open). The upper right histogram in Figure 4C delineates the respective isotype control (light gray, filled) that allows the subsequent marker position and identification of the CD107a+ subset in (B) and (C). In each histogram, the subtraction result ΔCD107a is given (% CD107a+ cells in NKAES-tumor cocultures minus % CD107a+ cells in NKAES-only cultures). Note that all experiments were performed with highly activated NKAES cells that exhibited a significant baseline CD107a expression. (D) Percentage of CD107a+ CD56+ cells in the indicated NK-cell subsets of KIR-KIRL mismatched (SNK14B, SNK15B, and SNK20B) or matched donors (SNK9A, SNK10P, and SNK21BC). Shown is the increase in CD107a expression of NALM-16 cocultured KIR+ NK-cell subsets normalized to the CD107a expression of the corresponding KIR− NK-cell subset. Filled black circle, KIR+ alloreactive subset in KIR-KIRL mismatched donors; open circle, KIR+ alloreactive subset in matched donors; light gray square, KIR+ nonalloreactive NK-cell subset. Data represents 1 experiment performed with the 6 indicated donors. n.s., not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/26/10.1182_blood-2014-05-572743/4/m_3914f4.jpeg?Expires=1769086109&Signature=40uvrz8kIJNZ-Hzn0UmJdYmiDWEmKubnQHKfErwS4Ho1fO8tVBVm9Glw8orEZOJfaGWjQ4gwcNfc2LCpGt4tSCq9Hvine5Y1V0xTDyYJpJDZWqpmffg3WV82L7g09qJ8OBwer2IwOF4nPKQWWs~AtsWyYk7RmPsKgCRgMgroNNiXEhIQqx7~UdRc-djA5T1L9-oPMzRfXCYYiuyN55RK9B1AjFDbHILZgpE~KnKCSkUZ8sfHaRE-15INxA-j5tnIrcu1BFCDuoKw~7BXsA6UPm1kqfNzoz9GMp4gl3grb9qDWeJ5YonFxgt0fAILPghxDDtCiQJK3Nqsn2ziQJ0y0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. The alloreactive KIR+ NK-cell subset of KIR-KIRL mismatched donors exhibits a superior ability for degranulation in response to pediatric BCP-ALL. (A-D) NKAES cells of KIR-KIRL mismatched (SNK14B, SNK15B, and SNK20B) or matched donors (SNK9A, SNK10P, and SNK21BC), respectively, were cocultured with NALM-16 or K562 cells. Shown is the percentage of CD107a+ CD56+ population in various alloreactive or nonalloreactive NK-cell subsets. (A) Exemplified gating strategy for the identification of NK-cell subsets in donor SNK15B. In relation to NALM-16 cells (Bw4/C1), the potentially alloreactive NK-cell population (upper left square) is represented by the cells expressing KIR2DL1/S1/S4 and/or KIR3DS1 (y-axis) but not KIR2DL2/L3/S2 or KIR3DL1 (x-axis). (Note that the combined staining with a KIR3DL1/S1 [Z27.3.7] and a KIR3DL1 [DX9] antibody enables the identification of the KIR3DL1−S1+ cell population that belongs to the alloreactive subset). (B-C) Representative original histogram data obtained from a KIR-KIRL mismatched (SNK15B) and a matched donor (SNK10P). Data in (B) shows CD107a staining in NKAES-only cultures (gray, open) and NKAES cells cocultured with NALM-16 cells (black, open). Data in (C) shows the respective stainings in coculture experiments with K562 (black, open). The upper right histogram in Figure 4C delineates the respective isotype control (light gray, filled) that allows the subsequent marker position and identification of the CD107a+ subset in (B) and (C). In each histogram, the subtraction result ΔCD107a is given (% CD107a+ cells in NKAES-tumor cocultures minus % CD107a+ cells in NKAES-only cultures). Note that all experiments were performed with highly activated NKAES cells that exhibited a significant baseline CD107a expression. (D) Percentage of CD107a+ CD56+ cells in the indicated NK-cell subsets of KIR-KIRL mismatched (SNK14B, SNK15B, and SNK20B) or matched donors (SNK9A, SNK10P, and SNK21BC). Shown is the increase in CD107a expression of NALM-16 cocultured KIR+ NK-cell subsets normalized to the CD107a expression of the corresponding KIR− NK-cell subset. Filled black circle, KIR+ alloreactive subset in KIR-KIRL mismatched donors; open circle, KIR+ alloreactive subset in matched donors; light gray square, KIR+ nonalloreactive NK-cell subset. Data represents 1 experiment performed with the 6 indicated donors. n.s., not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/26/10.1182_blood-2014-05-572743/4/m_3914f4.jpeg?Expires=1769686960&Signature=n21pXJjLHNyzeGH~zbwa~~4KcOoGdg58yO3Lq3ShqiXgwXxw0Jck8EkDd~0UGPsYz8GZAB6DEbud1FllZrSSnZpv32RDJtMwgC1Na2nsps4KNKwUSpUFo6z9wuQFZmi01r6iTvIccRxKkFDr1kdwkrknRa-NWgz2jhxkdS2gkd10dtlBM-wsezESmfJmmIeVcbefGgCDTNt0sbb1XAcOiPtZgKstTzeKk7w7UfVVkCQ9P0oHodd-Xc7Lwy~lizQkNn393gzAu0khjUgcA~YY2R3fuW019YGMODuceFrO92swd~nUYZGExEpma5C-wVMqDAkHWHpPkxTb8YfYkS27CA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)