Abstract
Langerhans cell histiocytosis (LCH) is a rare disease affecting people of any age, with widely variable clinical manifestations and different outcomes. The precise chain of events driving lesional granuloma formation has remained elusive for many years. There is evidence for inherited predisposition to and derangement of apoptosis and inflammation in lesional dendritic cells. Recently somatic BRAFV600E mutation in myeloid precursor dendritic cells was associated with the more aggressive form of the disease, although the same mutation in a more differentiated dendritic cell might drive a less aggressive disease. Whether this picture convincingly put LCH in the field of myeloid neoplasm remains to be determined. Altogether, these findings suggest that future therapeutic strategy might incorporate a screening of this genetic mutation for high-risk patients potentially suitable for target therapy.
Introduction
Langerhans cell histiocytosis (LCH) is a rare disease mainly seen in children, but it can present at any age.1,2 The clinical manifestations range from a solitary, asymptomatic osteolytic lesion called “eosinophilic granuloma” that tends to heal spontaneously to multiple osteolytic lesions of the skull, intriguingly associated with exophthalmos and diabetes insipidus (DI), formerly called “Hand-Schüller-Christian” disease. In addition, in a small proportion of patients, especially toddlers, an ominous form of LCH (once described as “Abt-Letterer-Siwe” disease) may run an acute course with dissemination to various organs other than bones (particularly liver and spleen), thus shaping a multisystem disease as life-threatening as childhood acute lymphoblastic leukemia. Based on solid pathology findings and on a bit of intuition, Lichtenstein suggested in 1953 that these three conditions were part of a common disease spectrum, and he coined the term histiocytosis X, conveying the message that dysregulated proliferation of a histiocytic cell was at the heart of the problem but that the trigger for this was unknown.3
The diagnosis of LCH is based on finding in a biopsy, usually of skin or bone, a granuloma consisting of pale histiocytic cells with infolded nuclei, eosinophils, and multinucleated giant cells. Staining of the histiocytic cells for CD1a (and CD207 langerin) has become part of the diagnosis (the demonstration of Birbeck granules by electron microscopy is no longer required).1,3 The morphology of individual lesions is so uniform as to make it impossible for the pathologist to know whether a biopsy was from a patient with unifocal or multifocal disease, from a child or an adult, and whether the disease was clinically indolent or life-threatening; thus, after more than half a century, Lichtenstein’s notion is fully vindicated.
Systemic manifestations of LCH
Skin and bone lesions are the most frequent but fortunately the least threatening manifestations of LCH. At the other end of the spectrum, massive liver involvement and dysfunction may lead to rapidly fatal outcome or, sometimes, to late postinflammatory fibrosis (sclerosing cholangitis). In a minority of these patients, liver transplantation may prove curative, but in many, the disease reactivates in the transplanted organ.4 Pulmonary involvement is one of the most puzzling features of LCH. Children may have disseminated interstitial pulmonary nodules and cysts that are responsive to systemic therapy.5 Conversely, isolated pulmonary LCH is by far the most frequent manifestation in adults. A correlation with cigarette smoking has been clearly documented. Yet, in most cases, smoking cessation is not sufficient to stop the inflammatory process, and these young adults may show progression to multicystic metaplasia and become oxygen dependent. In such patients, the role of LCH-directed chemotherapy remains unclear, because existing reports are on small series, and no prospective studies have been conducted.6 End-stage organ failure often requires lung transplantation, and here too, disease recurrence may take place.
DI, resulting from destruction of the supraoptic-paraventricular nuclei in which vasopressin is produced,7 occurs in about 12% to 15% of patients with disseminated LCH. Osteolytic lesions of the skull base and facial bones predispose to DI, likely because of local vascular dissemination. Once DI is established, reversal may be exceptional.8 Thus, DI can be a disabling sequela, and it may progress to multiple anterior pituitary hormone deficiency.9 A small number of patients with LCH, especially among those with DI, may develop bilateral symmetric alterations in the cerebellar gray matter, in the basal ganglia, and in the brainstem. Histopathology from cerebellar biopsies and from autopsies revealed neuronal loss, axonal degeneration, and a profound T-cell inflammation. Its pathogenesis remains unsolved, but propagation from long-standing granulomatous lesions of the craniofacial bones to the intracranial space, with stimulation of chemokine/cytokine tissue damage or initiation of an autoimmune response to brain components have been suggested.10 Neurodegenerative LCH may be devastating and, unfortunately, no effective therapy is available so far.
Treatment of LCH
LCH tends to run a favorable course in the large majority of patients; indeed, most patients with solitary lesions do not need treatment as long as the lesion remains solitary (although it must be acknowledged that even biopsy itself may exert some therapeutic effect and may speed up the healing process). However, in a minority of cases, the disease may be aggressive and even life threatening. For a long time, the management of patients was conducted on an empirical basis, reflecting the different views and the uncertainty that have prevailed regarding the nature of the disease. Leukemia-oriented chemotherapy was used in children with disseminated disease by the Austrian-German group during the 1980s, with favorable response. Conversely, based on the concept of an inflammatory disease, anti-inflammatory agents, especially steroids, were used by the British group; although this achieved disease control in most cases, children with chronic and/or reactivating LCH developed unacceptable side effects from long-lasting steroid exposure.11 Since the early 1990s, an international cooperative group of pediatric hematology-oncology specialists met the challenge of conducting 3 consecutive randomized trials in this orphan disease. Overall, these studies provided meaningful lessons: First, in LCH-I, which was a comparison of 6-month therapy with vinblastine or etoposide together with an initial 3-day pulse of prednisone, all patients with multisystem LCH (MS-LCH) were equivalent with respect to response, survival, disease reactivation, permanent consequences, and toxicity. However, early response and prevention of disease reactivation were inferior to results of the more aggressive (5-drug combination) and longer (12 months) DAL-HX 83 and DAL-HX 90 protocols, suggesting a need to intensify treatment.12,13 Second, in LCH-II, MS-LCH patients were stratified by risk (high risk being those with age <2 years and/or involvement of a risk organ such as liver, spleen, hematopoietic system, and lung) and were randomly assigned to the standard combination of prednisone and vinblastine vs additional etoposide. In both treatment arms, patients showed faster disease resolution and a higher survival rate than those in LCH-I, although the 44% reactivation rate was still high. Lack of advantage, together with its reported leukemogenic potential, caused the discontinuation of study of etoposide in patients with MS-LCH.14 Third, in the LCH-III successor study, the efficacy of increasing intensity by adding methotrexate in risk-organ patients (treated for 12 months) and prolonging initial intense therapy if only a partial response was achieved by 6 weeks was tested but did not provide a better outcome; otherwise, extending the duration of treatment to 12 months proved superior to 6 months in reducing the rate of disease reactivation in children who had achieved disease control.15
As a result of these trials, the former intuition, that patients with LCH should be stratified according to their very different risk of progression and treatment failure,16 has been validated. Patients with localized disease should be treated conservatively with very limited exceptions; patients with multisystem disease without involvement of vital organs should be treated with the combination of vinblastine and prednisone for a total duration of at least 12 months, which represents the standard of care in pediatric LCH, aimed at limiting the disease course and also permanent consequences. Yet there are still additional unmet clinical needs: about one third of patients who achieve complete control of the disease will suffer relapse during the following months, most often within the same tissue or organ(s) type(s) involved at presentation. Fortunately, whereas in childhood leukemia relapse is usually associated with very unfavorable prognosis, relapsed LCH is usually amenable to treatment with the same agents used before, or even with anti-inflammatory agents. For this reason, the term “reactivation,” which has been proposed since 1982,16 is now preferred to the term “relapse.”17 Beyond disease reactivation, a number of permanent consequences,16 including DI, neurodegeneration, sclerosing cholangitis, pulmonary failure, reduced linear growth and other endocrine dysfunctions, and bone deformities, represent quite a heavy burden for the quality of life of patients cured of LCH.17,18 But even more compelling is the remaining minority (20%) of MS-LCH patients with involvement of liver, spleen, bone marrow who fail to respond within 2 to 3 weeks, who still face an unacceptably high risk of early mortality in the range of 40%.12-15 In these patients, very intensive chemotherapy, similar to that used for acute myeloid leukemia, turned out to be useful,19 whereas the role of hematopoietic stem cell transplantation remains uncertain.20 But this is definitely the subset of patients for whom novel experimental therapies derived from improved knowledge of LCH pathogenesis appear warranted.
LCH: inflammatory or neoplastic?
Although it has only localized manifestations in most cases, LCH must be considered a systemic disease. Unlike leukemia, LCH does not seem to arise from the bone marrow and to spread elsewhere. The basic lesion, the granuloma—the morphology of which has already been exhaustively described by nineteenth-century pathologists—is characteristically enriched in dendritic cells (DCs), reminiscent of the typical pattern of tissue reaction to an intracellular pathogen of which the tuberculous granuloma is the prototype. Again, it is since the nineteenth century that an infectious origin of LCH had been surmised but never proven, and extensive epidemiological studies have failed to identify significant infectious associations.19 However, since the early 1990s, it has been claimed that clonal cell populations are present based on analysis through an X-linked marker (eg, the human androgen receptor assay; Humara) of lesional tissue from females with LCH.22 This finding is, per se, nonconclusive of cancer, because although cancer must be clonal, not every clonal population is cancer; indeed, clonal populations have been observed in benign disorders. Unexplained remission can occur in neoplasms, exceptionally even in widely metastatic cases; but spontaneous healing in LCH is not exceptional: on top of what is frequently seen in skin and bone lesion of children, the provocative observation that some young adult patients with pulmonary LCH remit after smoking cessation is one of the fascinating and incompletely understood features of LCH, suggesting that there may be many pathobiologic contributions to the disease process.
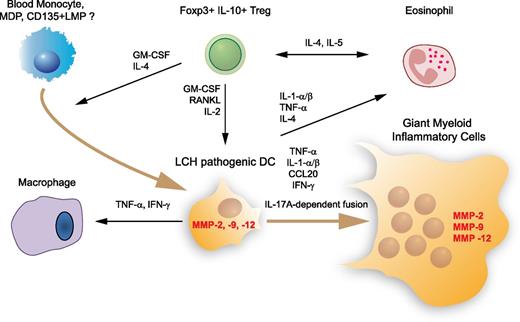
The fact that an animal model of LCH has been lacking for a long time has been a forceful stimulus to investigating pathogenesis by ex vivo studies and by looking for biomarkers. Clinical manifestations of LCH—aggressive chronic granuloma formation, bone resorption, and soft tissue lesions with occasional neurodegeneration—show similarities with those observed in other interleukin-17A (IL-17A) –related human diseases (Mycobacterium infection, Crohn’s disease, rheumatoid arthritis, and multiple sclerosis). In one study, peripheral blood samples from patients with LCH were put in culture; the resulting monocyte-derived DCs showed extended life span and propensity to undergo cell fusion, leading to the formation of multinucleated giant cells. These features make them excellent candidates to be at the origin of the characteristic granuloma. Remarkably, both of these properties—extended life span and propensity to fuse—were reproduced in culture by exposing normal control DCs to IL-17A; this phenomenon could be suppressed by exposure to anti-IL-17 antibodies and restored by re-exposure to IL-17A, suggesting an autocrine role for this cytokine with respect to granuloma formation.23 In the same study, patients with LCH were found to have high levels of IL-17A. However, a second group was unable to detect IL-17A messenger RNA (mRNA) or protein in samples from LCH patients.24 The authors suggested that the initial finding could be attributed to lack of specificity of the anti-IL-17A antibody. Subsequently, this laboratory failed to identify any cells in LCH lesions with IL-17A gene expression, concluding that evidence for IL-17A as a significant factor in LCH remained inadequate, thus beginning an IL-17 controversy in LCH.25 Yet the DC fusion activity of IL-17A in vitro was not dependent on an IL-17A antibody and has not been challenged, suggesting that IL-17A secreted by other cells might play a pathogenic role in LCH to foster tissue-aggressive giant myeloid inflammatory cell formation (Figure 1). Recently, Murakami et al26 confirmed higher levels of IL-17A in 38 patients with LCH compared with controls. Furthermore, Lourda et al27 found an increased frequency of IL-17A+ mononuclear cells in the bloodstream of LCH patients by using intracellular cytokine staining followed by flow cytometry analysis. The majority of the IL-17A+ cells were monocytes, which by reverse-transcription polymerase chain reaction (RT-PCR) showed higher levels of both IL-17A and retinoic acid orphan receptor C (RORC) mRNA, which is associated with higher disease activity. The finding that monocytes play a central role in this process appears to reconcile the controversy and agrees with the recent report of an immature marrow–based DC of origin in LCH.28
Major cell, cytokine, and protease players in LCH lesion. This simplified overview indicates the main cytokines, chemokines, and proteases found in an LCH lesion that may play a major role in cell recruitment, survival, fusion, and inflammatory and tissue-aggressive activities, supported by proinflammatory cytokines and matrix metalloproteinases (MMPs), respectively. The origin of the pathogenic DC in an LCH lesion is still a matter of debate. Recent results argue that pathogenic DCs do not arise from Langerhans cells but from accumulation of bone marrow–derived immature myeloid cells able to differentiate into DCs such as monocytes, macrophage and DC precursor (MDP), or CD135+ lympho-myeloid progenitor (LMP). CCL, chemokine (C-C motif) ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; RANKL, receptor activator of nuclear factor κ-B ligand; TNF, tumor necrosis factor; Treg, regulatory T cell.
Major cell, cytokine, and protease players in LCH lesion. This simplified overview indicates the main cytokines, chemokines, and proteases found in an LCH lesion that may play a major role in cell recruitment, survival, fusion, and inflammatory and tissue-aggressive activities, supported by proinflammatory cytokines and matrix metalloproteinases (MMPs), respectively. The origin of the pathogenic DC in an LCH lesion is still a matter of debate. Recent results argue that pathogenic DCs do not arise from Langerhans cells but from accumulation of bone marrow–derived immature myeloid cells able to differentiate into DCs such as monocytes, macrophage and DC precursor (MDP), or CD135+ lympho-myeloid progenitor (LMP). CCL, chemokine (C-C motif) ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; RANKL, receptor activator of nuclear factor κ-B ligand; TNF, tumor necrosis factor; Treg, regulatory T cell.
In spite of much recent progress on LCH that has just been reviewed, there remain three major questions to which we have only partial answers.
Why does a monocyte-derived DC become an LCH cell? The long-lasting question of a triggering pathogen in LCH has been revitalized by Murakami et al,29 who recently reported elevated amounts of Merkel cell polyomavirus DNA in the peripheral blood cells of 2 of 3 LCH patients with high-risk organ involvement, but not in the blood cells of 12 of 12 patients with low-risk LCH. Yet with lower viral loads, an elevated number of Merkel cell polyomavirus DNA sequences was detected in 12 LCH tissues compared with controls other than patients with dermatopathic lymphadenopathy. Previous studies of peripheral blood chromosomes showed that patients with LCH display an excess of spontaneous chromosomal breaks that are more evident during the acute phase of the disease30 ; these findings resemble the genomic instability induced by respiratory syncytial virus (RSV), hepatitis C virus (HCV), or Epstein-Barr virus (EBV).30,31
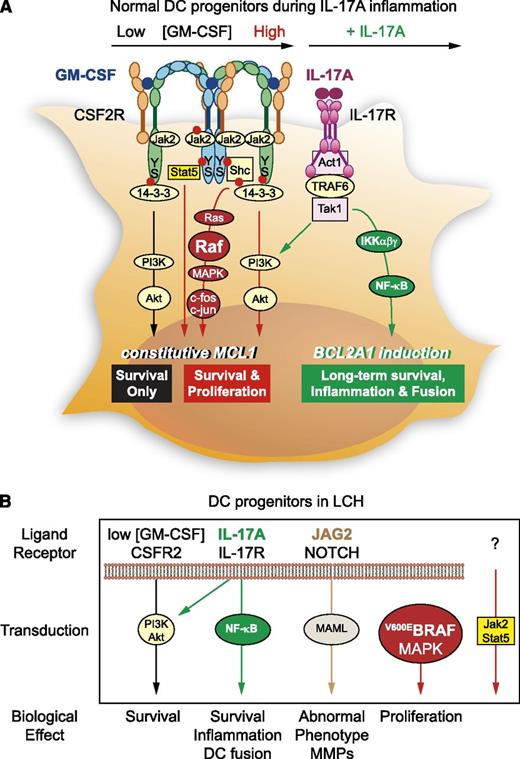
What are the key signals that drive monocyte-derived DCs to form a granuloma? Hutter et al32 showed that JAG2-mediated NOTCH activation confers phenotypic and functional aspects of LCH to DCs. Olsson et al33 found that monocyte-derived DCs treated with IL-17A express BCL2A1/BFL1, a prosurvival member of the Bcl-2 family. They also proposed that exposure to anti-IL-17A may decrease BFL1 and synergize with chemotherapy to eradicate LCH DCs.34 Identification of BRAFV600E mutation in 35 (57%) of 61 archived specimens of LCH tissue opened a novel research avenue in LCH.35 This finding was confirmed by a French group,36 which has not yet been able to document BRAFV600E in peripheral blood cells of patients with LCH, which would confirm that it is a somatic mutation within the lesional cells.37 In both studies, BRAFV600E did not seem to correlate with age, clinical presentation, or outcome. Yet those data were not conclusive: (1) only about 40% of LCH patients have somatic mutations of BRAF, (2) retroviral transduction of BRAFV600E in myeloid cells resulted in growth arrest and cell death, and (3) in genetically engineered mouse models, BRAFV600E mutation in mature Langerhans cells was insufficient to develop LCH.38 In a very recent study of 100 LCH lesions, of which 64 carried the BRAFV600E mutation within infiltrating CD207+ DCs, patients with active, high-risk LCH were found to carry BRAFV600E in circulating CD11c+ and CD14+ fractions and in bone marrow CD34+ hematopoietic cell progenitors, but the mutation was restricted to lesional CD207+ DCs in low-risk patients. In a new mouse model, the expression of conditional BRAFV600E enforced under the langerin promoter was sufficient to drive LCH-like disease. In summary, although expression of BRAFV600E in marrow DC progenitors was found to recapitulate the human high-risk LCH, BRAFV600E expression in differentiated DCs resembled low-risk LCH.39 On the basis of these findings, we propose classification of LCH as a myeloid neoplasia. Taking into account this collection of data, it is tempting to propose a working model for the impairment of transduction pathways driving LCH pathogenic DC development (Figure 2).40,41
Why do some people develop LCH? Studies of familial clustering of the disease have documented that about 1% of patients with LCH have another affected member in the family. When focusing on twin pairs, the rate of concordance is as high as 10% in nonidentical twins and an impressive 92% in identical twins.42,43 These data strongly point to a genetic component predisposing to LCH,42-46 even though efforts by Egeler et al to identify an LCH-associated locus through a genome-wide screening have not been successful.47 Interestingly, in this study, all patients had diploid genomes, which, if anything, casts additional doubt on the neoplastic origin of LCH.
Mechanisms of accumulation of LCH pathogenic DCs through proliferation and prolonged survival. (A) DC survival is basically regulated by exogenous concentration of GM-CSF on CSF2R. Low GM-CSF concentration (black) stimulates the selective activation of Ser585/14-3-3/PI-3 kinase (PI3K), the “survival only” pathway, and high GM-CSF concentration (red) can give rise to assembling of CSF2R chains40 in dodecamer, thus involving the Jak/STAT, Ras/mitogen-activated protein kinase (MAPK), and PI3K pathways (middle) downstream of Ser585 and Tyr577 phosphorylation (red bullet). In the IL-17A inflammatory microenvironment (green), monocyte-derived DC survival can be prolonged for weeks by IL-17A treatment.33 The TRAF6-dependent IL-17R transduction is able to activate both PI3K/Akt and nuclear factor κB (NF-κB) pathways.41 S, serine; Y, tyrosine. (B) In LCH patients, although low concentration of GM-CSF will normally induce PI3K/Akt (black), other molecular specificities turn DC survival and phenotype into aggressive myeloid cells: the presence of IL-17A activates both PI3K/Akt and NF-κB pathways (green), leading to increased survival, inflammation, and DC fusion. In addition, pathogenic LCH DCs express JAG2 and activate transduction downstream of its receptor NOTCH (brown),32 which may account for tissue destruction via MMP expression. BRAFV600E mutation will ensure constitutive activation of the MAPK pathway (red). It will be important in the future to explore the Jak2/Stat5 pathway, which may be absent in LCH DCs. From this recent knowledge, new therapeutic interventions in LCH may neutralize IL-17A, inhibit NOTCH and BRAFV600E/MAPK pathways, and possibly activate the Jak2/Stat5 pathway to reverse LCH DC phenotype toward normal DC phenotype until we better understand the origin of this impairment of DC differentiation.
Mechanisms of accumulation of LCH pathogenic DCs through proliferation and prolonged survival. (A) DC survival is basically regulated by exogenous concentration of GM-CSF on CSF2R. Low GM-CSF concentration (black) stimulates the selective activation of Ser585/14-3-3/PI-3 kinase (PI3K), the “survival only” pathway, and high GM-CSF concentration (red) can give rise to assembling of CSF2R chains40 in dodecamer, thus involving the Jak/STAT, Ras/mitogen-activated protein kinase (MAPK), and PI3K pathways (middle) downstream of Ser585 and Tyr577 phosphorylation (red bullet). In the IL-17A inflammatory microenvironment (green), monocyte-derived DC survival can be prolonged for weeks by IL-17A treatment.33 The TRAF6-dependent IL-17R transduction is able to activate both PI3K/Akt and nuclear factor κB (NF-κB) pathways.41 S, serine; Y, tyrosine. (B) In LCH patients, although low concentration of GM-CSF will normally induce PI3K/Akt (black), other molecular specificities turn DC survival and phenotype into aggressive myeloid cells: the presence of IL-17A activates both PI3K/Akt and NF-κB pathways (green), leading to increased survival, inflammation, and DC fusion. In addition, pathogenic LCH DCs express JAG2 and activate transduction downstream of its receptor NOTCH (brown),32 which may account for tissue destruction via MMP expression. BRAFV600E mutation will ensure constitutive activation of the MAPK pathway (red). It will be important in the future to explore the Jak2/Stat5 pathway, which may be absent in LCH DCs. From this recent knowledge, new therapeutic interventions in LCH may neutralize IL-17A, inhibit NOTCH and BRAFV600E/MAPK pathways, and possibly activate the Jak2/Stat5 pathway to reverse LCH DC phenotype toward normal DC phenotype until we better understand the origin of this impairment of DC differentiation.
In conclusion, from the clinical point of view, LCH is a rare disorder with widely variable manifestations. After decades in which its pathogenesis remained elusive, recent findings suggest that somatic mutation occurring in a bone marrow myeloid progenitor drive a neoplastic process, although the same somatic mutation occurring at a more differentiated stage drives a self-limiting, inflammatory disorder. The finding of BRAFV600E mutation in all concurrent pulmonary nodules48 suggests either multiple independent events or a true spread within the affected lungs. The monocyte/DC plays a pivotal role and might have been genetically predisposed42-46 to becoming overstimulated by an infectious trigger.
Until now, improved outcomes for patients with LCH have accrued from empirically adopted chemotherapeutic regimens; recent exciting advances in the pathogenesis of LCH may suggest incorporating in a therapeutic trial prospective validation of the association of BRAFV600E mutation with multisystem aggressive LCH and higher risk of treatment failure and exploring the use of first-line target therapy with vemurafenib49 in accurately defined subgroups of patients with LCH.
Acknowledgment
The authors thank Prof Lucio Luzzatto (Istituto Toscano Tumori, Firenze, Italy) for fruitful discussion and contribution to manuscript writing, and Associazione Italiana Ricerca Istiocitosi for supporting the research.
Authorship
Contribution: M.A. designed and wrote the manuscript; and C.D. contributed to data interpretation, drew the figures, and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maurizio Aricò, Istituto Toscano Tumori, V.le G. Pieraccini 6, Building 27/B, 50139 Firenze, Italy; e-mail: Maurizio.arico@ittumori.it.