Key Points
Loss of Bim accelerated the development and increased the incidence of plasmacytomas in Eμ-v-abl transgenic mice.
As in multiple myeloma, elevated expression of myc and cyclin D genes was common and p53 deregulation was rare.
Abstract
Mice susceptible to plasma cell tumors provide a useful model for human multiple myeloma. We previously showed that mice expressing an Eµ-v-abl oncogene solely develop plasmacytomas. Here we show that loss of the proapoptotic BH3-only protein Bim or, to a lesser extent, overexpression of antiapoptotic Bcl-2 or Mcl-1, significantly accelerated the development of plasmacytomas and increased their incidence. Disease was preceded by an increased abundance of plasma cells, presumably reflecting their enhanced survival capacity in vivo. Plasmacytomas of each genotype expressed high levels of v-abl and frequently harbored a rearranged c-myc gene, probably as a result of chromosome translocation. As in human multiple myelomas, elevated expression of cyclin D genes was common, and p53 deregulation was rare. Our results for plasmacytomas highlight the significance of antiapoptotic changes in multiple myeloma, which include elevated expression of Mcl-1 and, less frequently, Bcl-2, and suggest that closer attention to defects in Bim expression is warranted.
Introduction
Multiple myeloma (MM) is a fatal malignancy of plasma cells, for which effective treatment remains a challenge. To dissect the factors determining disease development and responsiveness to therapy, a number of mouse models for MM have been developed. Pioneering studies by Potter and his colleagues showed that plasma cell tumors arose with long latency but high incidence following injection of mineral oils into the peritoneal cavity of certain inbred mouse strains (eg, BALB/c and NZB).1 The plasmacytomas invariably carried a chromosome translocation that linked the c-myc gene with either the immunoglobulin heavy chain (IgH) or light chain (Igκ or Igλ) gene loci,2,3 and Ig gene regulatory sequences rendered c-myc expression constitutive in the affected B lymphoid cell and its clonal progeny.4
A transgenic plasmacytoma model using the v-abl transgene was developed in our laboratory.5 The v-abl gene, isolated from Abelson murine leukemia virus (A-MuLV), encodes a nonreceptor tyrosine kinase in which the N-terminal SH3 domain of c-Abl has been replaced with retroviral gag, rendering it plasma-membrane bound and constitutively active.6 Abelson virus primarily transforms pro-B and pre-B cells in vivo7 and, depending on the helper virus, also thymocytes.8 In contrast, three of four lines of transgenic mice expressing a v-abl oncogene linked to the Eµ-associated enhancer from the IgH locus developed plasmacytomas exclusively, and without the need for priming with pristane.5 Disease penetrance was high in the original (C57BL/6 × SJL) background (60% by 12 months of age) but later shown to vary with genetic background, being considerably higher in BALB/c than C57BL/6 mice (80-90% vs 10-20%, respectively, by 12 months).9,10 Susceptibility was polygenic, as in the mineral oil-induced plasmacytoma model11 and, as is the case for human MM, higher in males than females.10 Most Eµ-v-abl plasmacytomas harbored a rearranged myc gene, and plasmacytomas developed more rapidly in bitransgenic progeny of Eµ-v-abl and Eµ-myc mice.5 Infection with Abelson virus also accelerated plasmacytoma development in pristane-primed BALB/c mice.12
In a recently developed conditional transgenic model (Vk*MYC mice),13 in which expression of a human MYC gene inserted within a Vk transgene is dependent on removal of a stop codon through the activity of endogenous activation-induced deaminase (AID) in germinal center B cells, mice developed a slowly progressive expansion of plasma cells, with monoclonal antibodies evident in some animals as early as 20 weeks of age. As in human MM, the disease was largely confined to the bone marrow, whereas in Eµ-v-abl mice, the tumors were more disseminated, involving the mesenteric lymph node and Peyer’s patches, as well as the bone marrow.5
Although activation of myc increases cell growth and proliferation, it also increases the propensity of cells to undergo apoptosis under conditions of stress, eg, cytokine deprivation. We therefore hypothesized that inhibition of the stress-induced apoptosis pathway (also known as the intrinsic or mitochondrial cell death pathway), which is controlled by opposing factions of the Bcl-2 protein family,14 might accelerate plasmacytoma development. Here we report that plasmacytomagenesis in Eµ-v-abl mice was indeed accelerated by overexpression of antiapoptotic Mcl-1 or Bcl-2 and, even more so, by loss of the proapoptotic BH3-only protein Bim. The tumors arising in each of these genotypes had myc rearrangements. Thus, myc activation appears to be obligatory for the development of plasma cell tumors in Eµ-v-abl mice, and mutations that restrain apoptosis of plasma cells are synergistic with elevated Myc expression.
Materials and methods
Mice
Mouse lines used were Eμ-v-abl 40,5 bim−/− 266 Del,15 Eμ-bcl-2-36,16,17 vavP-mcl-1,18 Rag-1J−/−,19 and BlimpGFP,20 all on a C57BL/6J background and bred at the Walter and Eliza Hall Institute. Female Eμ-v-abl transgenic mice were used for all breeding experiments. Protocols were approved by Walter and Eliza Hall Institute’s Animal Ethics Committee. Mice were monitored for signs of tumor development and autopsied when sick. Tumor and tissue samples were fixed in 10% formalin prior to embedding in paraffin wax and staining with hematoxylin and eosin (H&E). Samples were also snap frozen on dry ice for protein and RNA extraction. Blood analysis was performed with an ADVIA 2120 analyzer (Siemens).
Cell culture
For the production of plasmablasts (PBs) in vitro, B cells were isolated from spleens using CD19 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured at 106/mL with 20 μg/mL lipopolysaccharide (Sigma-Aldrich, St Louis, MO) for 4 days in RPMI medium containing 10% fetal calf serum, 50 μM 2-mercaptoethanol (2-ME), 1 mM l-glutamine, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 1 mM sodium pyruvate.
For in vitro Nutlin 3a (Roche Nutley, Nutley, NJ) treatment, 1 to 2 × 106 plasmacytoma cells were isolated from ascites by flow cytometry and then cultured for 16 hours in RPMI medium containing 10% fetal calf serum, 50 μM 2-ME, and 1 mM l-glutamine with or without 10 µM Nutlin 3a. Cultures also contained 50 µM quinoline-Val-Asp-difluorophenoxymethylketone (Q-VD-OPh) to inhibit apoptosis.
Statistical analysis
GraphPad Prism (version 5.0a) was used to graph and statistically analyze data. For Kaplan-Meier survival curves, significance was determined using the log-rank (Mantel-Cox) test; for the remaining data, unpaired 2-tailed t tests were performed.
Flow cytometry, antibodies, cell death assays, serum immunoglobulin analysis, ELISPOT assays, polymerase chain reaction (PCR) analysis, and immunoblotting are detailed in supplemental Methods available on the Blood Web site.
Results
Eμ-v-abl transgene expression is mosaic
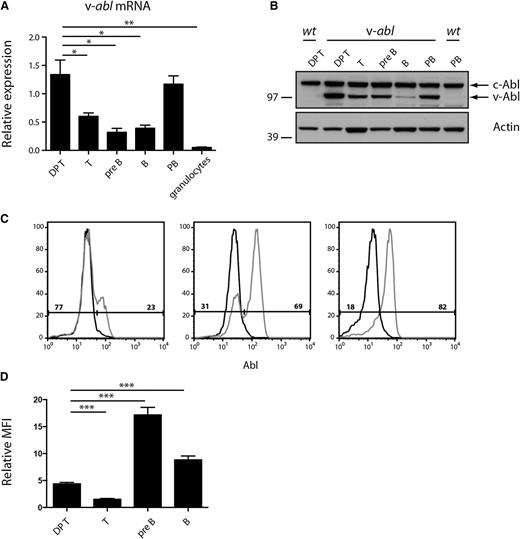
Quantitative PCR and western blot analyses performed on purified cell populations from Eμ-v-abl 40 (hereafter v-abl) mice indicated that the transgene was expressed in both the B- and T-lymphoid lineages and suggested that plasmablasts (PBs; generated in vitro by lipopolysaccharide [LPS] stimulation of B cells; B220−CD138+) and CD4+CD8+ (DP) thymocytes had the highest expression levels (Figure 1A-B). However, when assessed on an individual cell basis, by intracellular staining and flow cytometry, it became apparent that expression varied between animals, presumably due to epigenetic modulation. For example, the proportion of DP thymocytes expressing v-Abl protein varied from 20% to 85% between different transgenic animals (Figure 1C; data not shown), and for pre-B and B cells, it varied from 10% to undetectable (supplemental Figure 1). To compare the level of v-Abl protein expression between cell types, we compared the mean fluorescent intensity of wild-type (WT) and v-Abl-expressing cells in the different populations (Figure 1D). These data suggest that the small v-Abl-positive population of pre-B and B cells express higher levels of v-Abl than DP thymocytes. (Technological difficulties posed by their low frequency precluded a satisfactory analysis of plasma cells.) The mosaicism of transgene expression implies that the malignant clone will develop within an environment also populated by normal lymphoid cells, thereby more closely approximating normal tumor development than is the case in many transgenic models.
Eµ-v-abl transgene expression is mosaic. (A) Expression of v-abl mRNA in sorted lymphoid cell populations determined by quantitative reverse transcriptase-PCR and shown relative to the expression of the housekeeping gene hydroxymethylbilane synthase (HMBS). Mean ± standard error of the mean (SEM), n = 3. Statistical comparisons with DP thymocytes are shown, *P < .05, **P < .01, Student t test. DP T, CD4+CD8+ thymocytes; T, Thy1+ lymph node cells; pre-B, B220+ sIgD/IgM− bone marrow cells; B, B220+ lymph node cells; PB, plasmablasts (CD138+) produced by LPS stimulation of B cells in vitro; granulocytes, Mac1+Gr1+ bone marrow cells. (B) Western blot analysis of c-Abl and v-Abl protein expression in sorted lymphoid cell populations from WT and v-abl mice. Representative of 2 independent blots. Molecular weight (MW) markers are indicated (kDa). (C) Intracellular staining of DP thymocytes from WT (black curves) and v-abl (gray curves) mice using biotinylated anti-Abl antibody. As the antibody detects both c-Abl and v-Abl, cells expressing the transgene (right peak) have a higher level of total Abl protein. For the 3 v-abl mice shown here, 23%, 69%, and 82%, respectively, of the thymocytes express v-Abl protein, as indicated. (D) v-Abl expression level varies in different cell types. The relative mean fluorescent intensity (MFI) was determined by comparing the difference in MFI of WT and v-Abl expressing (right peak) cells in different lineages (relative MFI = MFI v-Abl-expressing cells/MFI WT cells). n = 12 mice from 3 independent experiments; mean ± SEM. Statistical comparisons with DP thymocytes are shown, ***P < .001, Student t test.
Eµ-v-abl transgene expression is mosaic. (A) Expression of v-abl mRNA in sorted lymphoid cell populations determined by quantitative reverse transcriptase-PCR and shown relative to the expression of the housekeeping gene hydroxymethylbilane synthase (HMBS). Mean ± standard error of the mean (SEM), n = 3. Statistical comparisons with DP thymocytes are shown, *P < .05, **P < .01, Student t test. DP T, CD4+CD8+ thymocytes; T, Thy1+ lymph node cells; pre-B, B220+ sIgD/IgM− bone marrow cells; B, B220+ lymph node cells; PB, plasmablasts (CD138+) produced by LPS stimulation of B cells in vitro; granulocytes, Mac1+Gr1+ bone marrow cells. (B) Western blot analysis of c-Abl and v-Abl protein expression in sorted lymphoid cell populations from WT and v-abl mice. Representative of 2 independent blots. Molecular weight (MW) markers are indicated (kDa). (C) Intracellular staining of DP thymocytes from WT (black curves) and v-abl (gray curves) mice using biotinylated anti-Abl antibody. As the antibody detects both c-Abl and v-Abl, cells expressing the transgene (right peak) have a higher level of total Abl protein. For the 3 v-abl mice shown here, 23%, 69%, and 82%, respectively, of the thymocytes express v-Abl protein, as indicated. (D) v-Abl expression level varies in different cell types. The relative mean fluorescent intensity (MFI) was determined by comparing the difference in MFI of WT and v-Abl expressing (right peak) cells in different lineages (relative MFI = MFI v-Abl-expressing cells/MFI WT cells). n = 12 mice from 3 independent experiments; mean ± SEM. Statistical comparisons with DP thymocytes are shown, ***P < .001, Student t test.
v-abl expression does not alter sensitivity to γ-irradiation
We wished to determine whether expression of the v-abl transgene affects sensitivity to apoptosis. In view of the mosaicism described above, it was only practicable to test this in thymocytes, which had the highest proportion of v-Abl-expressing cells. Thymocytes from 4 mice were suspended in medium lacking exogenous cytokines, exposed (or not) to γ-irradiation (either 2.5 or 10 Gy), and cultured for 8 h. As expected, thymocyte viability declined over time, particularly in the irradiated cultures (supplemental Figure 1B). Significantly, however, for each mouse, the proportion of v-Abl-expressing DP thymocytes did not change (although the values varied between individuals) (supplemental Figure 1C). The implication is that v-Abl protein neither enhances nor diminishes the sensitivity of DP thymocytes to γ-irradiation or cytokine deprivation in vitro.
Plasmacytomagenesis is accelerated when apoptosis is restrained
To determine the impact of antiapoptotic mutations on tumor development in v-abl mice, crosses were performed with mice that lack proapoptotic Bim (bim−/− line 266 Del)15 and with Eµ-bcl-2-36 (hereafter bcl-2tg) mice, which overexpress antiapoptotic Bcl-2 in both B- and T-lymphoid cells,17 and vavP-mcl-1(33) (hereafter mcl-1) mice, which overexpress antiapoptotic Mcl-1 in all hematopoietic lineages tested.18 All mice were on a C57BL/6 background, avoiding effects of differences in genetic background.9,10
Loss of Bim significantly accelerated tumorigenesis in v-abl mice (P < .0001; Figure 2A), as did overexpression of Mcl-1 or Bcl-2 (P = .01 and P = .0002, respectively) (Figure 2B-C), with loss of Bim having the greatest impact. Of note, all but one of the tumors were plasmacytomas (the exception was a CD138− splenic lymphoma comprised of B220+IgM+ B and CD4+ T cells in an mcl-1/v-abl mouse autopsied at day 355). Histologically, in each genotype, some plasmacytomas (20-30%) were comprised of mature plasma cells having eccentric nuclei with a “clock face” appearance and a large basophilic cytoplasm (Figure 2D), whereas other tumors contained less differentiated plasmacytoid cells (data not shown). Most mice (80-90%) presented with a grossly enlarged mesenteric lymph node, and tumor deposits were also found in the spleen, bone marrow, and, less frequently, the liver (supplemental Table 1). As in human MM, some v-abl mice developed kidney disease, characterized by renal deposition of immunoglobulin and neutrophil infiltration (Figure 2E; supplemental Table 1). When stained sections of affected tissues were graded for disease severity, only those from bim−/−v-abl mice had a greater tumor burden than v-abl mice (Figure 2F; data not shown), particularly in the spleen (P = .05) and bone marrow (P = .001). The tumors were transplantable in Rag-1−/− recipient mice (supplemental Table 2).
Inhibition of apoptosis accelerates plasmacytomagenesis. Kaplan-Meier survival curves in cohorts of (A) bim−/−v-abl (n = 24; P < .0001); (B) mcl-1/v-abl (n = 39; P = .013); and (C) bcl-2/v-abl (n = 47; P = 0.0002) mice and their v-abl littermates (n = 18, 39, and 57, respectively). (D-F) Histological analysis of moribund mice. H&E-stained sections from (D) a mesenteric lymph node tumor showing cells of classic plasmacytoma morphology (400× magnification) and (E) the kidney of a plasmacytoma-bearing mouse, showing the typical myeloma kidney characteristics, namely protein deposits and neutrophil infiltration in the tubules (200× magnification, photographed using a compound microscope; Axioplan 2; Zeiss). (F) H&E-stained sections of the organs from moribund v-abl (n = 10) and bim−/−v-abl (n = 12) mice were scored blind for the degree of disease infiltration on a scale of 0 to 3 (with 3 being 100% of the organ affected). Mean ± SEM, *P < .05, **P < .01, Student t test. (G) Analysis of plasmacytoma clonality by serum gel electrophoresis. In most cases a single paraprotein (arrowed) is apparent (compare with serum from WT); however, bim−/−v-abl #149 is biclonal.
Inhibition of apoptosis accelerates plasmacytomagenesis. Kaplan-Meier survival curves in cohorts of (A) bim−/−v-abl (n = 24; P < .0001); (B) mcl-1/v-abl (n = 39; P = .013); and (C) bcl-2/v-abl (n = 47; P = 0.0002) mice and their v-abl littermates (n = 18, 39, and 57, respectively). (D-F) Histological analysis of moribund mice. H&E-stained sections from (D) a mesenteric lymph node tumor showing cells of classic plasmacytoma morphology (400× magnification) and (E) the kidney of a plasmacytoma-bearing mouse, showing the typical myeloma kidney characteristics, namely protein deposits and neutrophil infiltration in the tubules (200× magnification, photographed using a compound microscope; Axioplan 2; Zeiss). (F) H&E-stained sections of the organs from moribund v-abl (n = 10) and bim−/−v-abl (n = 12) mice were scored blind for the degree of disease infiltration on a scale of 0 to 3 (with 3 being 100% of the organ affected). Mean ± SEM, *P < .05, **P < .01, Student t test. (G) Analysis of plasmacytoma clonality by serum gel electrophoresis. In most cases a single paraprotein (arrowed) is apparent (compare with serum from WT); however, bim−/−v-abl #149 is biclonal.
In confirmation of the histologic diagnosis, all tumors analyzed by flow cytometry (n = 48; all genotypes) had a plasma cell phenotype (CD138+B220−), although the levels of CD138 were variable, even within individual tumors (data not shown). As expected, electrophoresis revealed the presence of abundant discrete immunoglobulin bands in the sera of plasmacytoma-burdened mice (Figure 2G). Most of the tumors appeared to be monoclonal, in contrast to the oligoclonal tumors frequently seen in bi-transgenic Eμ-myc/Eμ-v-abl mice.5 Most plasmacytomas secreted IgA or IgG2b, with IgA being the most common isotype for all genotypes (supplemental Table 3). Whereas none of the 43 v-abl tumors analyzed in the previous study had secreted IgM,5 1 of 18 v-abl tumors and 3 of 12 bim−/− v-abl tumors analyzed in this study were IgM secretors. The difference in genetic background (C57BL/6 × SJL vs C57BL/6 in this study) may account for this difference. Sequencing of the IgH variable region (Igh-V) gene of 6 plasmacytomas did not identify any mutations (data not shown), suggesting the v-abl plasmacytomas arise from extrafollicular PBs, which undergo class switch recombination but not somatic hypermutation.21,22
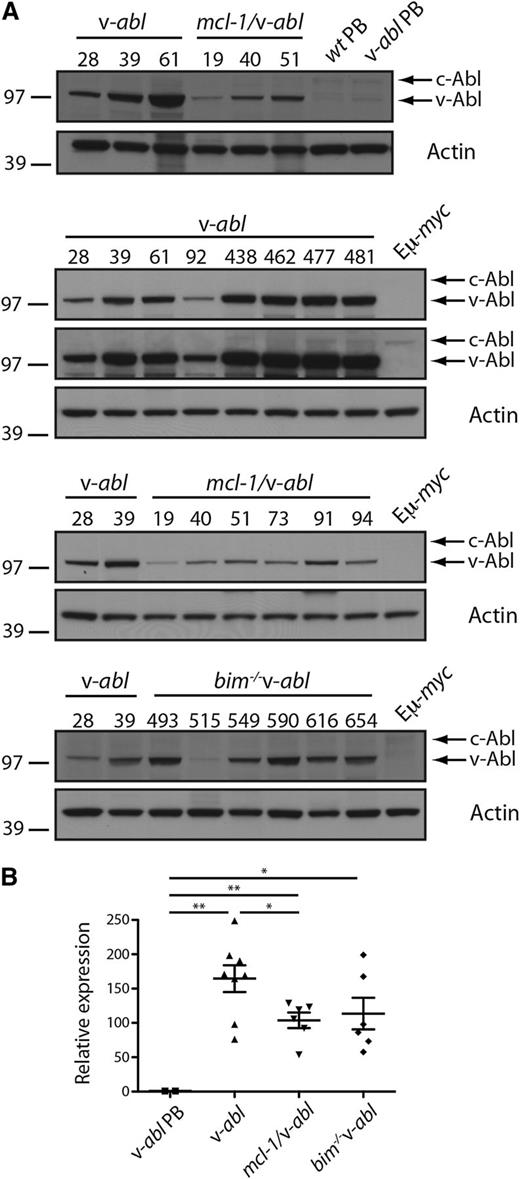
Expression of v-abl RNA and protein was readily detectable in all plasmacytomas analyzed (8 v-abl, 6 bim−/−v-abl, and 6 mcl-1/v-abl) and considerably higher than that of endogenous c-Abl. Of interest, expression was lower in mcl-1/v-abl plasmacytomas than in most v-abl and many bim−/− v-abl plasmacytomas (Figure 3A-B). Thus, upregulation of transgene expression is common during the process of malignant transformation, and overexpression of Mcl-1 appears to relieve this selection pressure.
v-abl transgene is highly expressed in plasmacytomas. (A) Western blot analysis of v-abl protein expression in plasmacytomas (whole tissue) from v-abl, mcl-1/v-abl, and bim−/− v-abl mice and PBs generated in vitro by LPS stimulation of B cells from WT and v-abl mice (Materials and methods) (note: the in vitro generated PB from v-abl mice contained very few v-abl expressing cells; data not shown). Two exposures are shown for the v-abl plasmacytomas to demonstrate that c-Abl can only be seen when v-Abl is overexposed. This is in contrast to Figure 1B, where levels of v-Abl and c-Abl were comparable and suggests that v-Abl expression is high in the plasmacytomas due to expression in all cells and upregulation. MW markers are indicated (kDa). (B) Real-time PCR analysis of v-abl mRNA in plasmacytoma cells from individual v-abl, mcl-1/v-abl, and bim−/− v-abl mice and PB generated in vitro from v-abl mice (see above). Expression was normalized to HMBS, and the data are expressed relative to PBs. Mean ± SEM, n = 6 to 8 independent tumors. *P < .05, **P < .01, Student t test.
v-abl transgene is highly expressed in plasmacytomas. (A) Western blot analysis of v-abl protein expression in plasmacytomas (whole tissue) from v-abl, mcl-1/v-abl, and bim−/− v-abl mice and PBs generated in vitro by LPS stimulation of B cells from WT and v-abl mice (Materials and methods) (note: the in vitro generated PB from v-abl mice contained very few v-abl expressing cells; data not shown). Two exposures are shown for the v-abl plasmacytomas to demonstrate that c-Abl can only be seen when v-Abl is overexposed. This is in contrast to Figure 1B, where levels of v-Abl and c-Abl were comparable and suggests that v-Abl expression is high in the plasmacytomas due to expression in all cells and upregulation. MW markers are indicated (kDa). (B) Real-time PCR analysis of v-abl mRNA in plasmacytoma cells from individual v-abl, mcl-1/v-abl, and bim−/− v-abl mice and PB generated in vitro from v-abl mice (see above). Expression was normalized to HMBS, and the data are expressed relative to PBs. Mean ± SEM, n = 6 to 8 independent tumors. *P < .05, **P < .01, Student t test.
Antiapoptotic mutations increase the frequency of plasma cells in preneoplastic v-abl mice
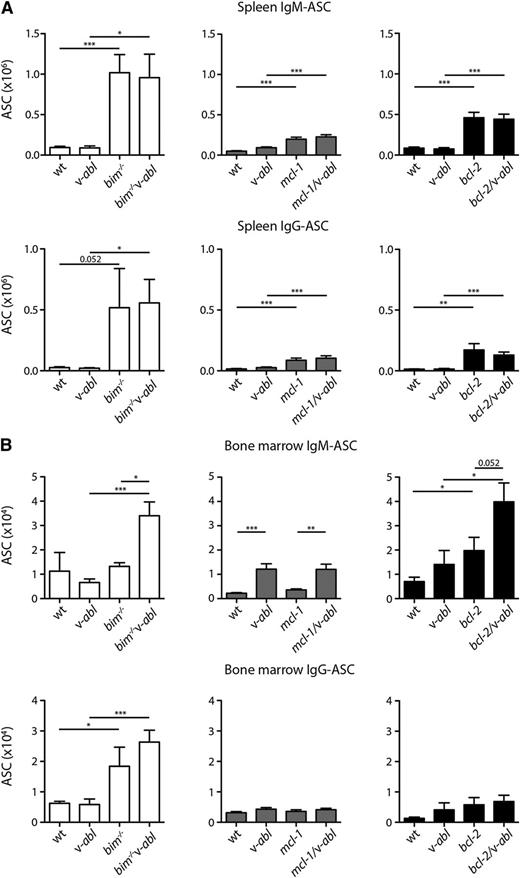
To explore the basis for the acceleration of plasmacytomagenesis, we enumerated the numbers of plasma cells in the spleen and bone marrow of healthy young (7-8 week old) mice of all genotypes using ELISPOT assays. In the spleen, the frequencies of IgM and IgG antibody-secreting cells (ASCs) were no higher in v-abl mice than in WT controls and, although plasma cell frequency increased in the v-abl mice bearing antiapoptotic mutations, particularly bim−/− v-abl mice, it was no higher than in the mice having only the antiapoptotic mutation (Figure 4A). In the bone marrow (Figure 4B), results were variable for v-abl vs WT mice, but ASCs were elevated in bim−/− (IgG-ASC), bim−/−v-abl (IgM- and IgG-ASC), bcl-2 (IgM-ASC), and bcl-2/v-abl (IgM-ASC) mice. We infer that the antiapoptotic mutations accelerated plasmacytomagenesis in v-abl mice by elevating the frequency of plasma cells, presumably by extending their lifespan in vivo. Mcl-1 is essential for the survival of plasma cells,23 and overexpression of Bcl-2 or Bcl-xL increases plasma cell frequency,24,25 as does loss of Bim.15 Of the antiapoptotic mutations investigated, loss of Bim causes the greatest increase in ASC numbers and correlates with the greatest acceleration in plasmacytomagenesis (Figure 2).
Inhibition of apoptosis increases the frequency of ASCs in preneoplastic v-abl mice. The total number of ASCs in the (A) spleen and (B) bone marrow (per femur) of healthy young mice (7-8 weeks) was determined by ELISPOT assay; total tissue cellularity is shown in supplemental Figure 2. bim−/−v-abl and littermates (white fill), n = 3 to 6; mcl-1/v-abl and littermates (gray fill), n = 10 to 12; bcl-2/v-abl and littermates (black fill), n = 8. Mean ± SEM. *P < .05, **P < .01, ***P < .001, Student t test. Statistical analysis for WT vs bim−/−v-abl and v-abl vs bim−/− are not shown.
Inhibition of apoptosis increases the frequency of ASCs in preneoplastic v-abl mice. The total number of ASCs in the (A) spleen and (B) bone marrow (per femur) of healthy young mice (7-8 weeks) was determined by ELISPOT assay; total tissue cellularity is shown in supplemental Figure 2. bim−/−v-abl and littermates (white fill), n = 3 to 6; mcl-1/v-abl and littermates (gray fill), n = 10 to 12; bcl-2/v-abl and littermates (black fill), n = 8. Mean ± SEM. *P < .05, **P < .01, ***P < .001, Student t test. Statistical analysis for WT vs bim−/−v-abl and v-abl vs bim−/− are not shown.
To explore whether the plasmacytomagenesis may also have been influenced by selection for changes in the expression of Bcl-2 family members, we analyzed their levels in nonmalignant PBs (generated in vitro) and v-abl, mcl-1/v-abl, and bim−/−/v-abl plasmacytomas by western blot (Figure 5; supplemental Figure 3; data not shown) and by quantitative PCR (supplemental Figure 4). No notable differences were detected for proapoptotic family members, apart from lower expression of bim (protein and RNA) in mcl-1/v-abl tumors. Mcl-1 expression in the tumors was comparable to that in normal PBs, except in mcl-1/v-abl tumors, where, as expected, it was elevated approximately two- to fivefold. Bcl-2 and A1 expression were very low or undetectable in most plasmacytomas, although readily apparent in PBs. However, irrespective of genotype, the level of Bcl-xL was higher in most plasmacytomas (14 of 20) than in normal PBs, suggesting that v-abl-driven plasmacytomagenesis selects for upregulation of Bcl-xL. Overexpression of Bcl-xL has been shown to accelerate plasmacytomagenesis in other transgenic mouse models.25,26
Expression of Bcl-2 family proteins in plasmacytomas. Western blot analysis of the indicated plasmacytomas was performed using lysates prepared from snap-frozen tissue. (A) Plasmacytomas were compared with PBs generated in vitro by LPS stimulation of B cells from WT and v-abl mice (Figure 3) and Eμ-myc tumor 48 (which contains a floxed Mcl-1 allele encoding an additional 13 amino acids79 ). The mcl-1 transgene produces a FLAG-tagged Mcl-1 slightly larger than endogenous Mcl-1.18 (B) Comparison of a panel of v-abl plasmacytomas. See also supplemental Figure 3 for western blots on panels of mcl-1/v-abl and bim−/−v-abl plasmacytomas. MW markers are indicated (kDa). The Bcl-2 antibody cross-reacted with additional proteins in tumor 61, an IgG2b producer. Bcl-xL expression was compared within and between these blots, and those in supplemental Figure 3, using ImageJ 1.48v software (http://imagej.nih.gov/ij/). The Bcl-xL expression level was normalized to actin and then expressed relative to v-abl #39, which was run on all blots. From this analysis, 14 of 20 plasmacytomas had higher Bcl-xL than the 2 PB samples tested.
Expression of Bcl-2 family proteins in plasmacytomas. Western blot analysis of the indicated plasmacytomas was performed using lysates prepared from snap-frozen tissue. (A) Plasmacytomas were compared with PBs generated in vitro by LPS stimulation of B cells from WT and v-abl mice (Figure 3) and Eμ-myc tumor 48 (which contains a floxed Mcl-1 allele encoding an additional 13 amino acids79 ). The mcl-1 transgene produces a FLAG-tagged Mcl-1 slightly larger than endogenous Mcl-1.18 (B) Comparison of a panel of v-abl plasmacytomas. See also supplemental Figure 3 for western blots on panels of mcl-1/v-abl and bim−/−v-abl plasmacytomas. MW markers are indicated (kDa). The Bcl-2 antibody cross-reacted with additional proteins in tumor 61, an IgG2b producer. Bcl-xL expression was compared within and between these blots, and those in supplemental Figure 3, using ImageJ 1.48v software (http://imagej.nih.gov/ij/). The Bcl-xL expression level was normalized to actin and then expressed relative to v-abl #39, which was run on all blots. From this analysis, 14 of 20 plasmacytomas had higher Bcl-xL than the 2 PB samples tested.
Plasmacytomagenesis in v-abl mice is associated with dysregulated expression of myc and cyclin D
Despite the acceleration of morbidity, plasmacytomagenesis in the bim−/− v-abl, bcl-2/v-abl, and mcl-1/v-abl mice remained relatively slow. Although the mosaic transgene expression (see above) undoubtedly reduced the probability of malignant transformation, it seemed likely that the emergence of a fully malignant clone required additional oncogenic mutation(s), as also implied by the (largely) monoclonal nature of the tumors.
Chromosomal rearrangements affecting the c-myc gene are a common feature of plasmacytomas1,27 and of MM.28 Most (7 of 11) of the v-abl plasmacytomas analyzed had a c-myc rearrangement detectable by Southern blot analysis, as reported previously,5 and the same was true of bim−/− v-abl (8 of 11) and mcl-1/v-abl (10 of 11) plasmacytomas (Figure 6A). All tested had elevated levels of myc mRNA (Figure 6B). These results underline the critical role of myc deregulation for the development of mouse plasmacytomas.
Activation of myc and cyclin D genes in v-abl plasmacytomas. (A) Southern blot analysis of EcoRI-digested DNA (20 μg) from v-abl, bim−/−v-abl and mcl-1/v-abl tumors using a c-myc exon 3 probe. (B) Real-time PCR analysis of cDNA prepared from frozen tumor samples and PBs generated in vitro. All v-abl plasmacytomas express high levels of c-myc mRNA, including those for which c-myc translocations were not apparent in A. Expression was normalized to HMBS, and the data are expressed relative to PBs. Mean ± SEM, n = 6 to 8 independent tumors. *P < .05, **P < .01, Student t test. (C) Real-time PCR analysis of cyclin D1, D2, and D3 mRNA in v-abl plasma cell tumors and PBs (generated in vitro). Expression was normalized to HMBS, and the data were expressed relative to v-abl PBs. Mean ± SEM, n = 6 to 8 independent tumors. *P < .05, **P < .01, Student t test. (D) Western blot analysis of cyclin D2 and D3 protein expression in v-abl tumors (whole tissue) and PBs (generated in vitro).
Activation of myc and cyclin D genes in v-abl plasmacytomas. (A) Southern blot analysis of EcoRI-digested DNA (20 μg) from v-abl, bim−/−v-abl and mcl-1/v-abl tumors using a c-myc exon 3 probe. (B) Real-time PCR analysis of cDNA prepared from frozen tumor samples and PBs generated in vitro. All v-abl plasmacytomas express high levels of c-myc mRNA, including those for which c-myc translocations were not apparent in A. Expression was normalized to HMBS, and the data are expressed relative to PBs. Mean ± SEM, n = 6 to 8 independent tumors. *P < .05, **P < .01, Student t test. (C) Real-time PCR analysis of cyclin D1, D2, and D3 mRNA in v-abl plasma cell tumors and PBs (generated in vitro). Expression was normalized to HMBS, and the data were expressed relative to v-abl PBs. Mean ± SEM, n = 6 to 8 independent tumors. *P < .05, **P < .01, Student t test. (D) Western blot analysis of cyclin D2 and D3 protein expression in v-abl tumors (whole tissue) and PBs (generated in vitro).
Many, if not all, MMs exhibit dysregulation of a cyclin D gene.29 We therefore compared the expression of cyclin D1, D2, and D3 mRNA in the plasmacytomas and nontransformed PBs using quantitative PCR (Figure 6C). Strikingly, cyclin D1 mRNA levels were >100-fold higher in the plasmacytomas than in the cultured PBs, which had very little expression. Cyclin D2 RNA was also elevated in the v-abl plasmacytomas, three- to fivefold, but cyclin D3 RNA was not. Western blotting showed an increased level of cyclin D2 in the v-abl tumors (Figure 6D), but cyclin D1 protein was not detectable with the antibodies available (see Discussion).
Status of the p53 pathway in v-abl plasmacytomas
Although myc deregulation enhances cell growth and proliferation, it also drives apoptosis when growth conditions become suboptimal.30,31 Myc activates expression of p19Arf, which binds to the Mdm2 E3 ubiquitin ligase and sequesters it in nucleoli, preventing Mdm2 from mediating proteosomal degradation of p53; the elevated p53 then drives apoptosis and cell cycle arrest.32 Accordingly, to overcome Myc-driven apoptosis, many Myc-driven tumors, such as Burkitt’s lymphomas and Eμ-myc lymphomas, carry mutations that inactivate the p19Arf/Mdm2/p53 pathway, either by loss of p19Arf, overexpression of Mdm2, or mutation of p53.33-37 We therefore analyzed p53 regulation in v-abl, mcl-1/v-abl, and bim−/− v-abl plasmacytomas.
p53 protein was barely detectable in either nonmalignant PBs or in the plasmacytomas (10 v-abl, 7 mcl-1/v-abl, and 6 bim−/−v-abl) (Figure 7A; data not shown). To determine whether the p53 pathway was in fact functional, sorted (CD138+) tumor cells were cultured for 16 hours in the presence or absence of the Mdm2 inhibitor Nutlin 3a.38 In 9 of the 10 tumors analyzed, Nutlin 3a treatment resulted in an increase in p53 protein and its target, the cyclin-dependent kinase inhibitor p21, presumably by blocking degradation of p53 by Mdm-2 (Figure 7B; data not shown). The 1 tumor that did not respond to Nutlin 3a treatment was v-abl 477, which also had a higher level of p53 in untreated cells and, unlike the other plasmacytoma cells, high p19Arf, suggestive of a p53 mutation. Sequence analysis of p53 DNA (exons 4-10) from 7 tumors (4 v-abl, 1 mcl-1/v-abl, and 2 bim−/−/v-abl) confirmed a lack of mutations in all but v-abl 477. The p53 mutation in v-abl 477, V271A, is in the DNA-binding domain and is equivalent to the V274A mutation that has been described in human cancers (IARC TP53 Database, http://p53.iarc.fr/). Together these data suggest that dysregulation of the p53 pathway is relatively uncommon in v-abl plasmacytomagenesis.
The p53 pathway is rarely dysregulated in v-abl plasmacytomas. (A) Western blot analysis of primary v-abl, mcl-1/v-abl, and bim−/−v-abl tumor tissue and Eμ-myc tumor 48 (positive control for mutant p53). (B) p53 activation in v-abl plasmacytoma cells following exposure to the Mdm-2 inhibitor Nutlin 3a. v-abl tumor cells (CD138+) isolated by flow cytometry from ascites in transplanted mice were cultured with 50 µM Q-VD-OPh alone (−) or in combination with 10 µM Nutlin 3a (+) for 16 h and then harvested for western blot analysis. Controls were Eµ-myc cell lines 22 (mutant for p53) and 45 (WT for p53). MW markers are indicated (kDa).
The p53 pathway is rarely dysregulated in v-abl plasmacytomas. (A) Western blot analysis of primary v-abl, mcl-1/v-abl, and bim−/−v-abl tumor tissue and Eμ-myc tumor 48 (positive control for mutant p53). (B) p53 activation in v-abl plasmacytoma cells following exposure to the Mdm-2 inhibitor Nutlin 3a. v-abl tumor cells (CD138+) isolated by flow cytometry from ascites in transplanted mice were cultured with 50 µM Q-VD-OPh alone (−) or in combination with 10 µM Nutlin 3a (+) for 16 h and then harvested for western blot analysis. Controls were Eµ-myc cell lines 22 (mutant for p53) and 45 (WT for p53). MW markers are indicated (kDa).
Discussion
This study set out to test whether inhibition of the Bcl-2-regulated apoptosis pathway facilitated malignant transformation of plasma cells driven by the Eµ-v-abl transgene. Indeed, in the absence of Bim, a major trigger for apoptosis in lymphoid cells,15,39 both the kinetics and penetrance of disease were enhanced considerably (Figure 2A). Bim is a potent tumor suppressor37,40 and plays an important role in regulating lymphoid homeostasis through its capacity to neutralize all 5 prosurvival Bcl-2-like proteins (Bcl-2, Bcl-xL, Bcl-w, A1, and Mcl-1) and activate Bax and Bak.14,41 Transgenic overexpression of the prosurvival proteins Mcl-1 or Bcl-2 also promoted plasmacytomagenesis significantly, but to a lesser extent than loss of Bim, presumably because neither Mcl-1 nor Bcl-2 can neutralize all BH3-only proteins.42,43 The Eμ-Bcl-2 transgene has also been shown to accelerate plasmacytoma development in Vk*MYC mice,13 as did coexpression of bcl-xL and myc transgenes late in B-lymphoid ontogeny.25,26,44
Remarkably, despite the increase in peripheral T- and B-lymphoid cells provoked by inhibition of apoptosis (supplemental Figure 2) and expression of the v-abl transgene in these compartments (Figure 1; supplemental Figure 1), with one exception (see above), no lymphomas arose in bim−/− v-abl, mcl-1/v-abl, or bcl-2/v-abl mice. It therefore seems likely that plasma cells are the primary targets for transformation in Eµ-v-abl mice (see below). The frequency of plasma cells was elevated in mice bearing the antiapoptotic mutations (Figure 4), presumably due to their reduced dependency on cytokine availability or a supportive stromal cell niche for survival and greater resistance to the endoplasmic reticulum (ER) stress provoked by secretion of large amounts of immunoglobulin. The increased number of plasma cells is probably a major factor contributing to the increased disease susceptibility evoked by loss of Bim or over-expression of Bcl-2 or Mcl-1. Enhanced survival capacity in the face of v-abl oncogene expression and other oncogenic lesions such as myc overexpression (see below) probably also contributes.
Irrespective of genotype, most v-abl-induced plasmacytomas analyzed contained a rearranged c-myc gene (Figure 6A),5 likely resulting from a chromosome translocation linking it to an immunoglobuin locus, and myc expression was higher than for normal PBs generated in culture (Figure 6B). It therefore seems likely that constitutive myc expression is a rate-limiting and perhaps obligate step for malignant transformation of plasma cells by v-abl. Most myc translocations associated with Burkitt’s lymphomas and mouse plasmacytomas depend on AID, the enzyme that initiates immunoglobulin class switch DNA recombination in B cells in germinal centers45 and extrafollicular foci. Thus, the probability of acquiring a myc translocation is highest at this stage in lymphoid ontogeny, and plasma cells emerging with a myc translocation may be highly susceptible to transformation by v-abl.
In addition to promoting proliferation and growth, activated myc and abl each transmit signals that induce apoptosis, which restrains their transforming potential. In both cases, the p19Arf/Mdm2/p53 pathway has been implicated.32,46,47 However, irrespective of genotype, the p53 pathway appeared to be unaffected in most v-abl plasmacytomas: the level of p53 was generally very low but increased, as expected, in the presence of the mdm2 inhibitor Nutlin 3a, as did p21, a p53 target (Figure 7). The exception was plasmacytoma 477, which expressed a higher level of p53 and readily apparent p19Arf; this tumor proved to be the only 1 of 7 sequenced that had a p53 mutation.
How do myc and v-abl synergize in transforming plasma cells? Presumably in several ways, because Myc regulates the transcription of a plethora of genes48,49 and, like other activated Abl tyrosine kinases, v-Abl upregulates multiple signal transduction pathways, including the Ras GTPase/mitogen-activated protein kinase (MapK), Rac GTPase, phosphoinositide 3-kinase (PI3K)/Akt (also known as protein kinase B), protein kinase C (PKC), and Janus kinase (Jak)/signal transducer and activator of transcription (Stat) pathways.50-52 Synergy between Myc and activated Ras, first shown for rat embryo fibroblasts,53 has also been established for myc-driven lymphomagenesis,54,55 and loss of signaling through the Ras/MapK pathway significantly delayed lymphoma development in Eµ-myc mice.56 Both Ras and Myc drive cell cycle progression, but each can promote aspects that are deficient in the other.57 Synergy between PI3K activation and Myc has recently been demonstrated in another Myc-driven lymphoma model.58
The basis for the predilection for plasma cell tumors rather than lymphomas in Eµ-v-abl mice remains unclear. One possibility is that higher v-abl expression is required to transform lymphocytes than plasma cells. Another is that the checkpoints guarding against v-abl transformation of T cells and more immature B-lineage cells are not as easily circumvented as in plasma cells. Pertinently, a retrovirus coexpressing v-abl and myc also induced solely plasma cell tumors, even following infection of purified pre-B cells, whereas retroviral expression of v-abl alone produced only pre-B lymphomas.59,60
Notable differences in cyclin D expression were apparent between v-abl-induced plasmacytoma cells and LPS-generated nontransformed PBs (Figure 6C-D). Strikingly, cyclin D1 mRNA was >100-fold higher in the plasmacytomas, although D1 protein was undetectable, perhaps because its level is still too low to detect with the available antibodies. Cyclin D2 transcripts were elevated approximately threefold in the plasmacytomas, and the level of cyclin D2 protein appeared even higher. Although upregulation of ccnd2 seems likely to be due to the constitutive Myc expression,61-63 ccnd1 is repressed by Myc.64 Therefore, upregulation of ccnd1 may be due to signaling through the Ras/MapK pathway,62 driven by v-abl.65,66 Although all D cyclins can drive G1 to S progression through binding to and activating the cyclin-dependent kinases CDK-4 and CDK-6, cyclin D1 also performs noncatalytic functions, including regulation of transcription, chromatin modification, and DNA damage responses, through interaction with other proteins.67 Plasmacytomas arising in bcl-xL/myc bi-transgenic mice also have significantly increased expression of cyclin D1 RNA but lower levels of cyclin D2 RNA.44 Ectopic expression of CCND1 is frequent in human mantle cell lymphoma, and transgenic studies have shown that Myc and cyclin D1 synergize in B lymphomagenesis in mice.68,69
It is instructive to compare the molecular features described here for v-abl mouse plasmacytomas with those of human MM, which is notable for its genetic diversity.29,70-72 Dysregulation of cyclin D genes, either directly by chromosome translocations (via 11q13 for CCND1, 12p13 for CCND2, or 6p21 for CCND3) or indirectly (via chromosomal translocations involving the MAF [musculoaponeurotic fibrosarcoma] gene or other mechanisms) is a feature of virtually all MMs and their precursors (monoclonal gammopathies of undetermined significance), indicative of an early predisposing change. Myc overexpression is common in MM, and myc chromosomal translocations, often involving loci other than immunoglobulin genes, occur in nearly 50% of cases, both treated and untreated.28 Upregulation of ABL expression is also a feature of MM.73 Our study highlighted the contribution of mutations that impair apoptosis in the development of plasma cell malignancies. Mutations of p53 are infrequent (∼5%) in newly diagnosed MM, but increase with progression. Furthermore, overexpression of MCL-1 or, less frequently, BCL-2 is associated with relapse and poor survival.74,75 Several studies implicate Bim in apoptosis of MM cells,76-78 and our results suggest that defects in Bim regulation may contribute to the development of MMs and/or their resistance to therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J. M. Adams, D. Tarlinton, and D. P. Lane for useful discussions and advice, J. M. Adams and D. Tarlinton for review of the manuscript, D. Tarlinton for technical advice, A. Kallies for kindly providing BlimpGFP mice, G. Siciliano, J. Mansheim, T. Camilleri, K. Trueman, K. Hughes, S. Allan, M. Robati, and J. Corbin for excellent technical assistance, and the institute’s Flow Cytometry Facility for skilled support.
This work was supported by NHMRC (Australia) program grants 461221, 1016701, and 1016647, National Cancer Institute grant CA43540, Leukemia and Lymphoma Society Specialized Center for Research grants 7015-02 and 7001-13, an NHMRC Career Development Award (to C.J.V.), and infrastructure support to Walter and Eliza Hall Institute from the National Health and Medical Research (NHMRC) Independent Research Institute Infrastructure Support Scheme and the Victorian State Government Operational Infrastructure Support.
This paper is dedicated to the memory of the late Michael Potter, who contributed so much to the field of plasmacytomagenesis and was a friend and mentor to S.C., and to our colleague the late Alan Harris, who codeveloped the Eμ-v-abl mice.
Authorship
Contribution: C.J.V. and S.C. conceived the studies, planned experiments, analyzed the data, and wrote the paper; A.S. performed an early cross between bcl-2 and v-abl (C57BL/6 × SJL) mice, which led to these studies; and P.W. analyzed plasmacytoma pathology.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suzanne Cory, Molecular Genetics of Cancer Division, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, VIC 3052, Australia; e-mail: cory@wehi.edu.au.