Key Points
There is a novel PIP3-independent and Gq-dependent Akt translocation mechanism in the platelets.
PAK constitutively associates with Akt, and possibly mediates its membrane translocation independently of PIP3.
Abstract
Akt is an important signaling molecule regulating platelet aggregation. Akt is phosphorylated after translocation to the membrane through Gi signaling pathways by a phosphatidylinositol-3,4,5-trisphosphate (PIP3)-dependent mechanism. However, Akt is more robustly phosphorylated by thrombin compared with adenosine 5′-diphosphate in platelets. This study investigated the mechanisms of Akt translocation as a possible explanation for this difference. Stimulation of washed human platelets with protease-activated receptor agonists caused translocation of Akt to the membrane rapidly, whereas phosphorylation occurred later. The translocation of Akt was abolished in the presence of a Gq-selective inhibitor or in Gq-deficient murine platelets, indicating that Akt translocation is regulated downstream of Gq pathways. Interestingly, phosphatidylinositol 3-kinase (PI3K) inhibitors or P2Y12 antagonist abolished Akt phosphorylation without affecting Akt translocation to the membrane, suggesting that Akt translocation occurs through a PI3K/PIP3/Gi-independent mechanism. An Akt scaffolding protein, p21-activated kinase (PAK), translocates to the membrane after stimulation with protease-activated receptor agonists in a Gq-dependent manner, with the kinetics of translocation similar to that of Akt. Coimmunoprecipitation studies showed constitutive association of PAK and Akt, suggesting a possible role of PAK in Akt translocation. These results show, for the first time, an important role of the Gq pathway in mediating Akt translocation to the membrane in a novel Gi/PI3K/PIP3-independent mechanism.
Introduction
Akt (also known as protein kinase B)1 is a 57-kDa serine/threonine kinase that contains a pleckstrin homology (PH) domain adjacent to a centrally located catalytic domain, which is connected to a short C-terminal domain2 . Akt is recruited to the membrane by the binding of its PH domain to the phosphatidylinositol 3-kinase (PI3K) products phosphatidylinositol-3,4-bisphosphate (PIP2) and phosphatidylinositol-3,4,5-trisphosphate (PIP3).3 At the membrane, Akt is phosphorylated at Thr308 by PDK1 and Ser473 by mTORC2.3-6 Forced membrane localization of Akt by the addition of a myristoylation motif at the amino terminus induces phosphorylation at both Thr308 and Ser473,7 indicating that membrane translocation is a crucial step for Akt activation. Although much is known about the translocation of Akt in other cell lines, the mechanism of Akt translocation to the membrane has never been studied in platelets.
Thrombin, generated at the site of vascular injury by extrinsic and intrinsic coagulation cascades, is an important agonist for platelet activation.8 Thrombin mediates its cellular effects primarily through G protein–coupled protease-activated receptors (PARs).9 PARs couple to the Gq and G12/13 pathways,1 and activation of platelets by thrombin or PAR-activating peptides causes Akt activation through secreted adenosine 5′-diphosphate (ADP).10,11 Secreted ADP activates the Gq-coupled P2Y1 receptor and the Gi-coupled P2Y12 receptor on platelets. Stimulation of platelets with thrombin results in Akt phosphorylation, and the ADP receptor P2Y12 is responsible for this Akt phosphorylation.12
The p21-activated kinases (PAKs) are a family of serine/threonine kinases known to be downstream effectors of Cdc42 and Rac.13,14 Binding of Cdc42–guanosine triphosphate (GTP) and Rac-GTP to the Cdc42/Rac interactive binding domain of PAK and autophosphorylation of serine/threonine residues in the regulatory domain leads to the opening of the molecule: transphosphorylation of threonine 423 in PAK1 or threonine 402 in PAK2.15-17 PAKs are the key regulators of actin polymerization and cell migration18 and are classified into two groups based on structural differences. Human platelets have been shown to express both group I PAKs (PAK1, PAK2, PAK3) and group II (PAK4).19 In thrombin-activated platelets, PAK is rapidly activated and plays a primary role in extensive cytoskeleton reorganization.20,21 It has been reported that the PAK signaling system plays an important role in activation of MEK/ERK, platelet spreading, and aggregation in thrombin-stimulated platelets.22 PAK is reported to interact with numerous proteins including Akt, PDK1, and PI3K in different cell lines.23-25 PAK’s function as a scaffolding protein expands the role of this protein in cellular functions. Although PAK is known to have noncatalytic scaffolding functions and is shown to associate and translocate Akt in other cell systems,23 the mechanisms of its activation and the scaffolding role in platelet functions are not clearly defined.
In this study, we investigated the molecular mechanisms of the quantitative differences in Akt phosphorylation by ADP and thrombin. We show that Akt is translocated to the membrane in a Gq-dependent mechanism that is independent of PIP3 formation. We have identified a possible scaffolding role of PAK in the translocation of Akt to the membrane in platelets. We show, for the first time, the constitutive association between PAK and Akt and a novel PIP3-independent translocation mechanism for Akt downstream of the Gq pathway in platelets.
Materials and methods
Materials
Apyrase (type VII), acetylsalicylic acid (aspirin), and YM-254890 were gifts from Yamanochi Pharmaceutical (Ibaraki, Japan). AR-C69931MX was a gift from Astra-Zeneca (Loughborough, UK). PF3758903 was a gift from Dr Jonathan Chernoff (Fox Chase Cancer Center, Philadelphia, PA). LY294002 was from Biomol Research Laboratories (Plymouth Meeting, PA). MRS 2179 and EHT 1864 were obtained from Sigma-Aldrich (St Louis, MO). Whatmann protein nitrocellulose transfer membrane was obtained from Fisher Scientific (Pittsburg, PA); LI-COR Odyssey Blocking Buffer was purchased from LI-COR Biosciences (Lincoln, NE). Antibodies to phospho-Akt 308 (4056) and phospho-Akt 473 (4058), phospho-PAK1/2 T423/T402 (2601), total Akt mouse monoclonal antibody (mAb) (2920), total Akt rabbit Ab (9272), Akt1 (2967), Akt2 (5239), and Akt3 (3788) were bought from Cell Signaling Technology (Beverly, MA). Total Akt mouse mAb (sc-5298), total PAK (sc-166887), β3-integrin (sc-14009), GRB14 (sc-20755), agarose-conjugated immunoglobulin (Ig)G (sc-2345), and AC-PAK (sc-881AC) antibodies were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Antibody to FREUD-1 (A300-285A) was obtained from Bethyl Laboratories (Montgomery, TX).
Preparation of human platelets
Blood was collected from informed healthy volunteers into one-sixth volume of acid/citrate/dextrose (1.5 g of citric acid, 2.5 g of sodium citrate, and 2 g of glucose in 100 mL of deionized water). Platelet-rich plasma was obtained by centrifugation at 250g for 20 minutes at ambient temperature and incubated with 1 mM aspirin for 30 minutes at 37°C. Platelets were isolated from plasma by centrifugation at 980g for 10 minutes at ambient temperature and resuspended in Tyrode’s solution pH 7.4 (138 mM sodium chloride, 2.7 mM potassium chloride, 2 mM magnesium chloride, 0.42 mM monosodium phosphate, 5 mM glucose, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 0.2 U/mL of apyrase, pH 7.4). The platelet count was adjusted to 2 × 109/mL for the membrane preparation and 1 × 109/mL for coimmunoprecipitation. Approval was obtained from the institutional review board of Temple University for these studies. Informed consent was provided prior to blood donation, in accordance with the Declaration of Helsinki.
Preparation of murine platelets
All mice were housed and maintained in a specific pathogen-free facility, and animal procedures were carried out in accordance with institutional guidelines after the Temple University Animal Care and Use Committee approved the study protocol. Age- and gender-matched wild-type animals were used as controls. Blood was drawn via cardiac puncture into one-tenth volume of 3.8% sodium citrate. Blood was then spun at 100g for 10 minutes and the platelet-rich plasma was separated. Red blood cells were mixed with 400 mL of 3.8% sodium citrate and spun for 10 minutes at 100g. Resulting platelet-rich plasma was combined with 1 μM prostaglandin E1 and centrifuged for 10 minutes at 400g. The platelet pellet was resuspended in Tyrode’s solution (pH 7.4) containing 0.2 U/mL of apyrase. Platelet counts were determined using a Hemavet 950FS blood cell counter (Drew Scientific, Dallas, TX). For this study, a platelet density of 1.5 × 109/mL was used.
Platelet aggregation
Platelet aggregation was measured using a lumiaggregometer (Chrono-Log, Havertown, PA) at 37°C under stirring conditions. A 0.5-mL sample of aspirin-treated washed platelets was stimulated with different agonists, and change in light transmission was measured. Platelets were preincubated with different inhibitors where noted before agonist stimulation. The chart recorder was set for 0.2 mm/s.
Preparation of platelet membrane fractions
Platelets were stimulated with agonists in the presence of inhibitors or antagonists/ vehicles, and the reaction was stopped using 2X Halt Protease and Phosphatase inhibitor Cocktail solution (Pierce, Rockford, IL) in Tyrode’s solution. Platelets were lysed by 4 freeze/thaw cycles and centrifuged at 1500g for 10 minutes at 4°C to pellet unlysed cells. Supernatants were ultracentrifuged at 100 000g for 30 minutes at 4°C. The supernatant (the cytosolic fraction) was removed. The pellet, containing the membranes and cytoskeleton, was resuspended in 100 μL of 1% Triton X-100. Samples were centrifuged at 15 000g for 10 minutes at 4°C to pellet down the cytoskeleton. Membrane-rich supernatant was collected, and an equal volume of 2X sample buffer was added. Protein estimation was performed using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Ten micrograms of protein was loaded on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel for separation and transferred to nitrocellulose membranes for specific immunoblotting.
Western blot analysis
Platelets were stimulated with agonists in the presence of inhibitors or vehicles for the appropriate time under stirring conditions at 37°C. Samples were prepared, and SDS-PAGE and western blotting were performed as described previously.26
Coimmunoprecipitation
Activation of washed platelets (1.5 × 109/mL) was stopped using equal volumes of chilled 2X NP-40 lysis buffer (50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 100 mM sodium chloride, 2% NP-40, 2 mM ethyleneglycoltetraacetic acid, and 100 mL of 2X Halt Protease and Phosphatase Cocktail solution) (Pierce, Rockford, IL). Samples were rocked for 30 minutes at 4°C and centrifuged at 10 000g for 10 minutes at 4°C to remove unlysed cells. Twenty microliters of agarose-conjugated normal rabbit IgG or agarose-conjugated rabbit PAK polyclonal IgG was added to the samples and incubated overnight on rocker at 4°C. Samples were centrifuged at 5000g for 30 seconds at 4°C to pellet down agarose beads. Agarose beads were then washed 3 times using 1X NP-40 lysis buffer and washed once using phosphate-buffered saline. Proteins were solubilized in 4X sample buffer, separated by SDS-PAGE, and transferred to nitrocellulose membrane.
Statistical analysis
Each experiment was repeated at least 3 times. Results are expressed as mean ± SEM with number of observations. Data were analyzed and graphs were plotted using KaleidaGraph software. The total Akt or phospho-Akt band density was first normalized using β3-integrin (for membrane fraction) or β-actin (whole cell lysate) as the lane-loading control. The unstimulated value was then subtracted from all other values. The highest value thus obtained was then taken as 100%, and rest of the samples were converted into percentages of the highest value. Significant differences were determined using the Student t-test. P values <.05 were considered significant.
Results
PAR agonists induce more robust Akt phosphorylation and membrane translocation than ADP in platelets
In platelets, Akt phosphorylation is known to depend on Gi stimulation by secreted ADP.12 Thrombin-receptor agonists, however, cause more robust Akt phosphorylation than ADP in both human (Figure 1A) and murine platelets (Figure 1B), although ADP is known to activate PI3K and is required for thrombin-induced Akt phosphorylation. We investigated the mechanism of this robust phosphorylation by thrombin-receptor agonists because the reason for such robust phosphorylation was not clear.
PAR agonist causes a more robust Akt phosphorylation and translocation in platelets than ADP. Washed human platelets (A) and murine platelets (B) were stimulated with 2MeSADP (100 nM) or AYPGKF (500 μM) for 2 minutes, and phosphorylation of Ser473 (S473) residue on Akt was evaluated by western blot analysis of whole cell lysates. Densitometric analysis of the western blot shows the percentage of phosphorylated Akt Ser473 normalized to β-actin. Phosphorylation (Ser473) and translocation of Akt was evaluated by western blot and densitometric analysis of membrane fractions prepared by ultracentrifugation of human (C) or mouse (D) platelets stimulated either with 100 nM 2MeSADP or 500 μM AYPGKF. β3-integrin was used as the lane-loading control as well as a membrane marker. Western blot shows Akt phosphorylation and translocation in whole cell lysate (E) and platelet membrane fraction (F) after human platelets were stimulated with different concentrations of 2MeSADP (30, 50, and 100 nM) or AYPGKF (50, 100, 250, and 500 μM) for 2 minutes in an aggregometer. Densitometric analysis is represented as a percentage of phospho-Akt S473 normalized to β-actin or Akt translocated to the membrane normalized with β3-integrin at different concentrations of 2MeSADP and AYPGKF. The western blot analysis shown is representative of 3 independent experiments using anti-Akt mouse mAb (Cell Signaling Technology).
PAR agonist causes a more robust Akt phosphorylation and translocation in platelets than ADP. Washed human platelets (A) and murine platelets (B) were stimulated with 2MeSADP (100 nM) or AYPGKF (500 μM) for 2 minutes, and phosphorylation of Ser473 (S473) residue on Akt was evaluated by western blot analysis of whole cell lysates. Densitometric analysis of the western blot shows the percentage of phosphorylated Akt Ser473 normalized to β-actin. Phosphorylation (Ser473) and translocation of Akt was evaluated by western blot and densitometric analysis of membrane fractions prepared by ultracentrifugation of human (C) or mouse (D) platelets stimulated either with 100 nM 2MeSADP or 500 μM AYPGKF. β3-integrin was used as the lane-loading control as well as a membrane marker. Western blot shows Akt phosphorylation and translocation in whole cell lysate (E) and platelet membrane fraction (F) after human platelets were stimulated with different concentrations of 2MeSADP (30, 50, and 100 nM) or AYPGKF (50, 100, 250, and 500 μM) for 2 minutes in an aggregometer. Densitometric analysis is represented as a percentage of phospho-Akt S473 normalized to β-actin or Akt translocated to the membrane normalized with β3-integrin at different concentrations of 2MeSADP and AYPGKF. The western blot analysis shown is representative of 3 independent experiments using anti-Akt mouse mAb (Cell Signaling Technology).
Activation of Akt by extracellular stimuli is a multistep process involving its translocation and phosphorylation. Hence, we investigated whether there are differences in the kinetics of translocation of Akt by thrombin and ADP that could account for the differences in its phosphorylation. Under stirring conditions in an aggregometer at 37°C, we stimulated washed human platelets with AYPGKF (a PAR-4 agonist) or 2MeSADP (an ADP-receptor agonist) and analyzed membrane fractions. There was more Akt translocated to the membrane, as well as more Akt phosphorylation in membrane fractions, after stimulation with AYPGKF compared with 2MeSADP in human platelets (Figure 1C) and murine platelets (Figure 1D). Stimulation of platelets with different concentrations of 2MeSADP and AYPGKF resulted in less Akt phosphorylation in whole cell lysates (Figure 1E) and translocation to the membrane (Figure 1F) at almost all concentrations of 2MeSADP. No Akt phosphorylation was observed only at the lowest concentration of AYPGKF (50 μM). In addition, the lowest concentration of AYPGKF induced a similar amount of translocation as did the highest concentration of 2MeSADP, with the robust increase correlating with increasing concentration of AYPGKF.
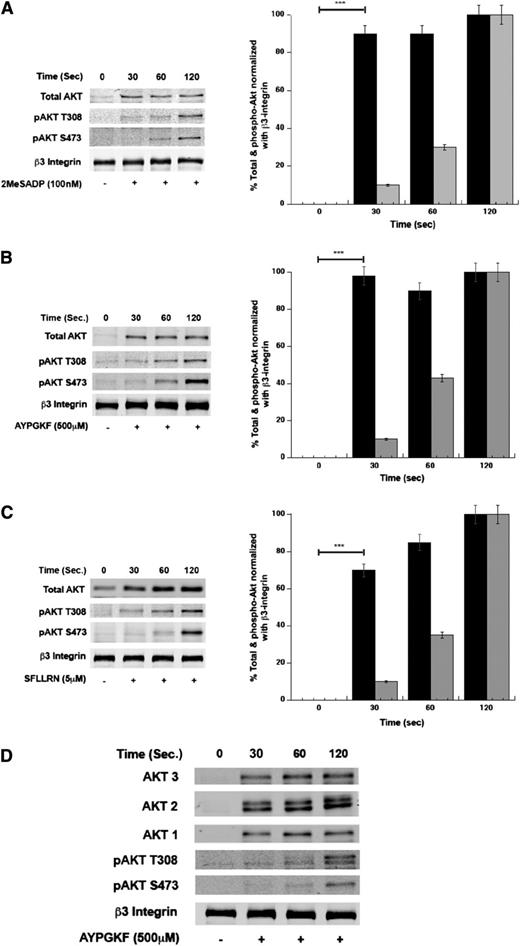
To determine the kinetics of Akt translocation downstream of P2Y receptors and PARs, we stimulated washed human platelets with 2MeSADP (Figure 2A) or with the thrombin-receptor-activation peptide AYPGKF (Figure 2B) or SFLLRN (Figure 2C), and evaluated the translocation and phosphorylation of Akt to the membranes. As observed in Figure 2, there was a rapid increase in Akt translocation after stimulation with all the agonists, as early as 30 seconds after stimulation, but the level of phosphorylation at Ser473 and Thr308 on Akt increased at approximately 2 minutes. Together, these results suggest that PAR agonists induce more Akt translocation to the membrane in platelets than ADP and that Akt translocation is an early event that has similar kinetics of translocation downstream of PARs and ADP receptors.
PAR agonist induces translocation and phosphorylation of Akt in platelets. Washed human platelets were stimulated with 2MeSADP (100 nM) (A), AYPGKF (500 μM) (B), or SFLLRN (5 µM) (C) for different time periods. Akt translocation and phosphorylation (Ser473 and Thr308) were evaluated by subjecting platelet membrane fractions to SDS-PAGE. β3-integrin was used as the lane-loading control. (D) Membrane fractions from platelets stimulated with AYPGKF were probed with anti-Akt isoform-specific antibodies. The western blot analysis shown is representative of 3 independent experiments using anti-Akt mouse mAb (Santa Cruz Biotechnologies).
PAR agonist induces translocation and phosphorylation of Akt in platelets. Washed human platelets were stimulated with 2MeSADP (100 nM) (A), AYPGKF (500 μM) (B), or SFLLRN (5 µM) (C) for different time periods. Akt translocation and phosphorylation (Ser473 and Thr308) were evaluated by subjecting platelet membrane fractions to SDS-PAGE. β3-integrin was used as the lane-loading control. (D) Membrane fractions from platelets stimulated with AYPGKF were probed with anti-Akt isoform-specific antibodies. The western blot analysis shown is representative of 3 independent experiments using anti-Akt mouse mAb (Santa Cruz Biotechnologies).
Contribution of Gq signaling to Akt translocation in platelets
It is known that PARs are coupled to Gq and G12/13 pathways.1 We thus examined whether the Gq or G12/13 pathway regulates Akt translocation. We analyzed the role of the Gq pathway in Akt translocation by using YM-254890, a Gq-selective inhibitor. Platelet membrane fractions revealed that both translocation and phosphorylation were abolished in the presence of YM-254890 (Figure 3A), suggesting that the Gq pathway plays an important role in Akt translocation. We confirmed these results by using Gq knockout mice. Consistent with the human platelet data, Akt translocation was abolished in Gq knockout murine platelets stimulated with AYPGKF in contrast to that of wild-type littermates (Figure 3B). These results thus suggest that the Gq pathway downstream of PARs is essential for translocation of Akt in platelets. The results also suggest that the G12/13 pathway does not play any role in Akt translocation, because in the absence of Gq, G12/13 by itself could not cause Akt translocation.
Contribution of Gq signaling to Akt translocation in human and murine platelets. (A) Washed human platelets were stimulated with AYPGKF (500 μM) in the presence or absence of 50 nM YM254890, a Gq inhibitor. The effect on Akt translocation and phosphorylation (Ser473 and Thr308) was evaluated by using western blot analysis of platelet membrane fractions using anti-Akt mouse mAb (Santa Cruz Biotechnologies). β3-integrin was used as the lane loading control. (B) Washed wild-type (WT) and Gαq-deficient (knockout [KO]) murine platelets were stimulated at 37°C for 2 minutes with AYPGKF (500 μM). Equal amounts of proteins from membrane fractions were analyzed for Akt translocation by western blot analysis using anti-Akt rabbit polyAb (Cell Signaling Technology). (C) Washed human platelets were stimulated with 2MeSADP (100 nM) in the presence or absence of 50 nM YM254890. The effect on Akt translocation and phosphorylation (Ser473 and Thr308) was evaluated by using western blot analysis of platelet membrane fractions using anti-Akt mouse mAb (Santa Cruz Biotechnologies) in panels A and C, and anti-Akt rabbit polyAb (Cell Signaling Technology) in panel B. The western blot analysis shown is representative of 3 independent experiments.
Contribution of Gq signaling to Akt translocation in human and murine platelets. (A) Washed human platelets were stimulated with AYPGKF (500 μM) in the presence or absence of 50 nM YM254890, a Gq inhibitor. The effect on Akt translocation and phosphorylation (Ser473 and Thr308) was evaluated by using western blot analysis of platelet membrane fractions using anti-Akt mouse mAb (Santa Cruz Biotechnologies). β3-integrin was used as the lane loading control. (B) Washed wild-type (WT) and Gαq-deficient (knockout [KO]) murine platelets were stimulated at 37°C for 2 minutes with AYPGKF (500 μM). Equal amounts of proteins from membrane fractions were analyzed for Akt translocation by western blot analysis using anti-Akt rabbit polyAb (Cell Signaling Technology). (C) Washed human platelets were stimulated with 2MeSADP (100 nM) in the presence or absence of 50 nM YM254890. The effect on Akt translocation and phosphorylation (Ser473 and Thr308) was evaluated by using western blot analysis of platelet membrane fractions using anti-Akt mouse mAb (Santa Cruz Biotechnologies) in panels A and C, and anti-Akt rabbit polyAb (Cell Signaling Technology) in panel B. The western blot analysis shown is representative of 3 independent experiments.
Because the ADP receptor P2Y1 is also coupled to Gq, we analyzed the role of Gq in the Akt translocation after stimulation with 2MeSADP. As shown in Figure 3C, YM-254890 did not affect the Akt translocation induced by 2MeSADP, suggesting that Gq does not play an important role in the Akt translocation downstream of ADP receptors.
PI3K activity is not required for Akt translocation in platelets
It has been shown that Akt phosphorylation requires signaling events downstream of Gi activation, involving PI3K.12 In addition, it was proposed that Akt translocates to the plasma membrane by binding to PIP3 generated by PI3K.3 The role of PI3K and the Gi pathway in Akt translocation, however, has not been studied in platelets. Thus, we examined the role of PI3K and Gi pathway in Akt translocation by using pharmacologic and genetic approaches. Immunoblot analysis of membrane preparations from platelets stimulated with either the PAR-4 agonist AYPGKF (Figure 4A) or the PAR-1 agonist SFLLRN (Figure 4B) in the presence of the pan-PI3K inhibitor LY294002 showed complete inhibition of Akt phosphorylation but failed to affect translocation of Akt to the membrane. 2MeSADP-induced Akt translocation, however, was significantly inhibited in the presence of LY294002 (Figure 4C). Similar results were obtained with 100 nM Wortmannin, another pan-PI3K inhibitor (data not shown), suggesting that PI3K is essential for Akt translocation downstream of ADP receptors but not PARs.
Effect of PI3K activity/Gi pathway on Akt translocation in human and murine platelets. Washed human platelets were stimulated with AYPGKF (500 μM) (A), SFLLRN (5 μM) (B), or 2MeSADP (100 nM) (C) in the absence or presence of LY294002 (25 μM; a pan-PI3K inhibitor). (D) Washed human platelets were stimulated with AYPGKF (500 μM) in presence or absence of ARC69931MX (100 nM; a P2Y12 antagonist). The effects of inhibitor/antagonist on Akt translocation and phosphorylation were determined by western blot analysis. The data shown are representative of 3 experiments. (E) WT and P2Y12-deficient (knockout [KO]) murine platelets were stimulated at 37°C for 2 minutes with AYPGKF (500 μM). Equal amounts of proteins from membrane fractions were analyzed for Akt translocation by western blot analysis. The western blot analysis shown is representative of 3 independent experiments using anti-Akt rabbit polyAb (Cell Signaling Technology) in panels A and E, and anti-Akt mouse mAb (Santa Cruz Biotechnologies) in panels B-D.
Effect of PI3K activity/Gi pathway on Akt translocation in human and murine platelets. Washed human platelets were stimulated with AYPGKF (500 μM) (A), SFLLRN (5 μM) (B), or 2MeSADP (100 nM) (C) in the absence or presence of LY294002 (25 μM; a pan-PI3K inhibitor). (D) Washed human platelets were stimulated with AYPGKF (500 μM) in presence or absence of ARC69931MX (100 nM; a P2Y12 antagonist). The effects of inhibitor/antagonist on Akt translocation and phosphorylation were determined by western blot analysis. The data shown are representative of 3 experiments. (E) WT and P2Y12-deficient (knockout [KO]) murine platelets were stimulated at 37°C for 2 minutes with AYPGKF (500 μM). Equal amounts of proteins from membrane fractions were analyzed for Akt translocation by western blot analysis. The western blot analysis shown is representative of 3 independent experiments using anti-Akt rabbit polyAb (Cell Signaling Technology) in panels A and E, and anti-Akt mouse mAb (Santa Cruz Biotechnologies) in panels B-D.
To support our hypothesis that PARs can induce Akt translocation independently of the Gi signaling pathway, we used the Gi-coupled P2Y12 receptor antagonist AR-C69931MX and stimulated platelets with AYPGKF (Figure 4D). In the presence of AR-C69931MX, the phosphorylation of Akt was abolished, but translocation of Akt was not affected. We further confirmed the PI3K activity/Gi-independent Akt translocation downstream of PARs by using P2Y12 knockout murine platelets. Consistent with the human data, Akt translocation was unperturbed in P2Y12 knockout murine platelets stimulated with AYPGKF, whereas phosphorylation of Akt was abolished compared with wild-type murine platelets (Figure 4E). These results suggest that Gi does not play a major role in Akt translocation. Gi activation, however, is necessary for Akt phosphorylation, as shown previously.12 Together, these results strongly suggest that Akt translocation occurs independently of PI3K activity and that the Gi signaling pathway is downstream of PARs.
Evaluation of scaffolding proteins in Akt translocation to the membrane
To elucidate the mechanism through which Akt is translocated to the membrane in a PIP3-independent manner, we examined the membrane translocation of three different Akt scaffolding proteins: FREUD1, PAK, and GRB14.23,28,29 Among the three proteins, only PAK is translocated to the membrane in platelets stimulated with AYPGKF. Although FREUD1 and GRB14 are expressed in platelets, they did not translocate to the membrane (supplemental Figure 1).
PAR-mediated Gq signaling plays a role in PAK translocation to the membrane
The time course of PAK translocation and phosphorylation was then followed in AYPGKF-activated platelets (Figure 5A). The kinetics of PAK translocation and phosphorylation correlate with that of Akt, which suggests that PAK may play a role in translocation of Akt to the membrane downstream of PARs. Considering that Akt translocation by PAR agonists is mediated through the Gq pathway, we investigated whether the Gq pathway regulates PAK translocation also. We observed that the translocation of PAK was abolished in the presence of YM254890, indicating that Gq plays a role in PAK translocation (Figure 5B) and that G12/13 is not necessary for PAK translocation. These results suggest that translocation of both PAK and Akt is mediated through similar Gq-dependent pathways downstream of PARs.
Role of Gq signaling pathways in PAK translocation in response to AYPGKF in human platelets. (A) Washed human platelets were stimulated with AYPGKF (500 μM) for different time periods. Translocation and phosphorylation of PAK were evaluated by subjecting platelet membrane fractions to SDS-PAGE analysis. β3-integrin was used as the lane loading control. (B) Washed human platelets were stimulated with AYPGKF (500 μM) in the presence or absence of 50 nM YM254890, and the effect on PAK translocation and phosphorylation was evaluated using western blot analysis of platelet membrane fractions. β3-integrin was used as the lane loading control. The western blot analysis shown are representative of 3 independent experiments.
Role of Gq signaling pathways in PAK translocation in response to AYPGKF in human platelets. (A) Washed human platelets were stimulated with AYPGKF (500 μM) for different time periods. Translocation and phosphorylation of PAK were evaluated by subjecting platelet membrane fractions to SDS-PAGE analysis. β3-integrin was used as the lane loading control. (B) Washed human platelets were stimulated with AYPGKF (500 μM) in the presence or absence of 50 nM YM254890, and the effect on PAK translocation and phosphorylation was evaluated using western blot analysis of platelet membrane fractions. β3-integrin was used as the lane loading control. The western blot analysis shown are representative of 3 independent experiments.
PAK interaction with Akt facilitates membrane translocation of Akt
Recent studies have reported that PAK plays a central role in thrombin-mediated platelet activation and in platelet physiological functions.19 In NIH 3T3 cells, it has been demonstrated that PAK regulates the efficiency, localization, and specificity of the PDK1-Akt pathway by acting as a scaffolding protein for Akt.23 Although the relationship between PAK and Akt has been reported previously in other cell lines, it has not been studied in platelets. We investigated whether PAK binds to Akt in AYPGKF-stimulated human platelets. We found that Akt coimmunoprecipitated with PAK in both unstimulated and AYPGKF-stimulated whole cell lysates (Figure 6A), suggesting for the first time that Akt constitutively associates with PAK in human platelets.
Role of PAK and Rac1 in Akt translocation. (A) Washed human platelets were stimulated for 1 minute at 37°C with 500 μM AYPGKF (AYP). Platelet lysates were immunoprecipitated (IP) with agarose-conjugated PAK rabbit polyclonal IgG, and samples were probed for coimmunoprecipitating Akt using anti-Akt mouse mAb (Cell Signaling Technology). (B) PAK1 knockout (KO) murine platelets were stimulated with 500 μM AYPGKF, and membrane fractions were subjected to SDS-PAGE. Western blots were then probed for anti-Akt, anti-phospho S473 Akt, and anti-PAK1 antibodies. β3-integrin was used as the lane loading control. (C) Washed human platelets were stimulated with AYPGKF (500 μM) in the presence or absence of 50 μM EHT1864, and the effect on PAK translocation and phosphorylation was evaluated using western blot analysis of platelet membrane fractions. β3-integrin was used as the lane loading control. The western blot analysis shown is representative of 3 independent experiments.
Role of PAK and Rac1 in Akt translocation. (A) Washed human platelets were stimulated for 1 minute at 37°C with 500 μM AYPGKF (AYP). Platelet lysates were immunoprecipitated (IP) with agarose-conjugated PAK rabbit polyclonal IgG, and samples were probed for coimmunoprecipitating Akt using anti-Akt mouse mAb (Cell Signaling Technology). (B) PAK1 knockout (KO) murine platelets were stimulated with 500 μM AYPGKF, and membrane fractions were subjected to SDS-PAGE. Western blots were then probed for anti-Akt, anti-phospho S473 Akt, and anti-PAK1 antibodies. β3-integrin was used as the lane loading control. (C) Washed human platelets were stimulated with AYPGKF (500 μM) in the presence or absence of 50 μM EHT1864, and the effect on PAK translocation and phosphorylation was evaluated using western blot analysis of platelet membrane fractions. β3-integrin was used as the lane loading control. The western blot analysis shown is representative of 3 independent experiments.
To elucidate the role of PAK in Akt translocation, platelets were treated with the PAK inhibitor PF3758903.19 The translocation of Akt, as well as of PAK, was intact in the presence of the PAK inhibitor (supplemental Figure 2). We further analyzed Akt translocation in platelets derived from PAK1-deficient mice. Membrane fractions obtained from murine platelets stimulated with AYPGKF showed that Akt translocation and phosphorylation were unaffected in PAK1-deficient platelets as compared with wild-type platelets (Figure 6B).
Because it was shown earlier that PAK interacts with GTP-bound Rac1 localized in membranes of thrombin-stimulated platelets,22,30 we investigated the role of Rac1 in PAK-Akt translocation. We incubated platelets with the Rac1 inhibitor EHT186431 and stimulated them with AYPGKF. As shown in Figure 6C, the translocation of PAK and Akt was significantly inhibited but not completely abolished in the presence of 50 μM EHT1864, suggesting that Rac1 might play an important role in PAK translocation.
Discussion
Akt has been shown to be activated by various agonists, including ADP, epinephrine, and thrombin in platelets.10,32-34 Previous studies from our laboratory have shown that thrombin and thrombin-receptor-activating peptides require Gi stimulation through secreted ADP to cause Akt phosphorylation.11,12 Although ADP-dependent Gi activation is required for Akt phosphorylation by both PARs and P2Y12 receptors, it was observed that 2MeSADP induced less Akt phosphorylation than thrombin or thrombin-receptor-activating peptides in platelets. Hence, it is conceivable that there might be some potentiating contribution of G protein signaling downstream of PARs to Akt phosphorylation. Akt activation involves two steps: translocation to the membrane and subsequent phosphorylation by PDK1 and mTORC2.4,6 We hypothesize that the enhanced Akt phosphorylation by PARs could be through a difference in Akt translocation. In particular, we focused on the potentiating effect of Gq and G12/13 pathways on the Akt translocation to the membrane downstream of PARs.
In support of our hypothesis, we observed that AYPGKF caused more Akt translocation to the membrane compared with 2MeSADP, which could account for the differences in the extent of phosphorylation by these two agonists. Indeed, the lowest concentration of AYPGKF caused a similar extent of translocation and phosphorylation of Akt as the highest concentration of 2MeSADP. The translocation of Akt to the membrane occurs rapidly, as early as 30 seconds, whereas the phosphorylation occurs at a later time point of 2 minutes, indicating the importance of Akt translocation as an essential step for its activation. The blocking of the Gq pathway by using YM-254890 or in Gq null mice platelets completely abolished AYPGKF-induced Akt translocation. In the presence of a P2Y12 antagonist or in P2Y12 null platelets, however, AYPGKF-induced translocation of Akt was unaffected. These results suggest that the Gq pathway is important for Akt translocation and indicate that G12/13 does not play any role in Akt translocation after PAR stimulation. In contrast, the Gq-coupled P2Y1 ADP receptor is not essential for Akt translocation because the inhibition of Gq downstream of 2MeSADP stimulation failed to inhibit Akt translocation. This failure may be due to a Gi/PI3K-dependent Akt translocation downstream of the P2Y12 receptor. Furthermore, PAR-mediated Akt translocation occurs primarily through Gq pathways, although secreted ADP could contribute to Akt translocation through a PI3K-dependent manner to a minor extent. Thus, the increased phosphorylation of Akt after PAR activation is due to the higher potency of PAR agonists to activate Gq (stronger Gq signaling downstream of PARs compared with P2Y1), resulting in more recruitment of Akt to the membrane.
Earlier studies in other cell lines and in vitro assays showed that Akt, via its PH domain, binds to the phospholipid PIP3.35,36 Generation of PIP3 by PI3K in response to receptor activation results in the Akt-PH domain binding to PIP3 and in Akt recruitment to the membrane.35-37 This membrane translocation is thought to cause a conformational change in Akt, allowing its phosphorylation and subsequent activation.37-40 In platelets, it is well known that Akt is phosphorylated downstream of the Gi pathway in a PI3K-dependent manner.12 It was also suggested that Akt translocation is PI3K dependent. It was shown that Akt phosphorylation, in response to thrombin and thrombin-related peptides in platelets, is through secreted ADP, which activates PI3K downstream of the P2Y12 receptor.10-12 This role may explain the delay in phosphorylation of Akt by PAR agonists, but the inhibition of PI3K did not affect Akt translocation in the present study. Our results challenge the established paradigm that the PI3K product PIP3 is required for Akt translocation. Thus, we suggest that although translocation events are regulated by the Gq pathway downstream of PARs, Gi signaling mediates the Akt phosphorylation events in platelets, as established in our previous studies.12 In contrast, PI3K is important for the Akt translocation downstream of the ADP receptors. We believe, however, that both Gq- and Gi/PI3K-dependent pathways contribute to the Akt translocation downstream of the ADP receptors, because inhibition of PI3K could not abolish ADP-induced Akt translocation completely.
To elucidate the mechanism by which Akt translocates to the membrane downstream of the Gq pathway, we probed for scaffolding proteins that are known to associate with Akt in different cells, such as FREUD1, GRB14, and PAK.23,28,29 Of the three proteins, only PAK translocates to the membrane in a Gq-dependent manner, suggesting a possible role of PAK in mediating Akt translocation by Gq-dependent pathways. PAK is the well-known downstream effector of the small GTPases, Rac1 and Cdc42. In cultured cells, binding of activated GTP-bound Rac1/Cdc42 to the Cdc42/Rac interactive binding domain of PAK recruits PAK to the cellular membrane where these GTPases are localized. PAK is also activated downstream of Rac1 and Cdc42 in platelets stimulated with different agonists.19,20 PAK plays an important role in platelet filopodia and lamellipodia formation, as well as in platelet aggregation, secretion, and thrombus formation.19,20,22 In NIH 3T3 cells, a previously unrecognized function of PAK was demonstrated; that is, PAK was shown to be a scaffolding protein that mediates the translocation of Akt to the membrane.23 These scaffolding functions account for the kinase-independent role of PAK. Other aspects of the close relationships between PAK and Akt have been demonstrated previously. For example, Akt phosphorylates and moderately activates PAK1, resulting in PAK1 dissociation from Nck and focal adhesions.41 It was therefore suggested that the activation process is bidirectional and that there may be a positive-feedback loop between PAK and Akt.
Evidence shown in the present study suggests a possible role of PAK in Akt translocation. First, there is a constitutive association between PAK and Akt in human platelets. Second, the kinetics of Akt and PAK translocation downstream of the Gq pathway to the membrane are similar. Hence, we suggest a possible role of PAK as a scaffold protein for Akt, mediating its translocation downstream of the Gq pathway in a novel PIP3-independent manner. PAK1 knockout murine platelets showed similar levels of translocation of Akt as in wild-type platelets, suggesting that other isoforms of PAK might take over the function of PAK1 in its absence. Because PAK2 knockout mice die early in embryogenesis due to multiple developmental abnormalities, we could not evaluate the role of PAK2. It is possible that PAK1 might take over the function of PAK2 in PAK2-null mice. In addition, the PAK inhibitor could not abolish the Akt translocation because the inhibitors will inhibit only the kinase activity of PAK but not its kinase-independent scaffolding function. It was also of interest to address how PAK translocates to the membrane in platelets. It was shown earlier that PAK interacts with GTP-bound Rac1 localized in membranes of thrombin-stimulated platelets.22,30 We also show here that Rac1 plays a major role in PAK translocation using a Rac1 inhibitor; however, the nonspecific effects of this inhibitor could not be ruled out. In addition, the possibilities of other mechanisms of PAK translocation remain to be investigated. One mechanism could be due to the presence of a Gβγ-binding domain in PAK that can interact with the Gβγ subunit of activated G protein–coupled receptors42
In conclusion, as outlined in Figure 7, we demonstrated that Gq, but not Gi, signaling is essential for Akt translocation by thrombin and thrombin-receptor-activating peptides in platelets. We also showed for the first time a novel PIP3-independent Akt translocation mechanism downstream of Gq in platelets. Furthermore, we showed that PAK and Akt are constitutively associated, suggesting that this complex translocates to the membrane after stimulation of PARs and that this event might be essential for Gq-mediated Akt translocation in platelets.
Proposed model depicting PAK-mediated Akt translocation to the membrane downstream of the Gq pathway in platelets. Activation of the Gq pathway results in translocation of the PAK-Akt complex to the membrane via interaction of PAK with membrane-localized Rac1 in a novel PIP3-independent mechanism. Akt, after translocation, is phosphorylated by PDK1 and mTORC2 by the Gi pathway through secreted ADP.
Proposed model depicting PAK-mediated Akt translocation to the membrane downstream of the Gq pathway in platelets. Activation of the Gq pathway results in translocation of the PAK-Akt complex to the membrane via interaction of PAK with membrane-localized Rac1 in a novel PIP3-independent mechanism. Akt, after translocation, is phosphorylated by PDK1 and mTORC2 by the Gi pathway through secreted ADP.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Leslie Parise, University of North Carolina, Chapel Hill, for providing the PAK1 null mice on C57BL/6 background, originally obtained from Dr Jonathan Chernoff.
This work is supported by National Institutes of Health National Heart, Lung, and Blood Institute grants HL93231 and HL118593 (S.P.K.) and National Cancer Institute grant R01 CA148805 (J.C.).
Authorship
Contribution: R.B., B.K.M., and S.P.K. designed the experiments; R.B. and B.K.M. performed the experiments; R.B., B.K.M., C.A.D., and S.P.K. analyzed and interpreted the data; R.B. wrote the manuscript; and J.C. provided PAK inhibitor and PAK1-knockout mice, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satya P. Kunapuli, Sol Sherry Thrombosis Research Center, Temple University, Room 414 MRB, 3420 N Broad St, Philadelphia, PA 19140; e-mail: spk@temple.edu.
References
Author notes
R.B. and B.K.M. contributed equally to this study.


![Figure 3. Contribution of Gq signaling to Akt translocation in human and murine platelets. (A) Washed human platelets were stimulated with AYPGKF (500 μM) in the presence or absence of 50 nM YM254890, a Gq inhibitor. The effect on Akt translocation and phosphorylation (Ser473 and Thr308) was evaluated by using western blot analysis of platelet membrane fractions using anti-Akt mouse mAb (Santa Cruz Biotechnologies). β3-integrin was used as the lane loading control. (B) Washed wild-type (WT) and Gαq-deficient (knockout [KO]) murine platelets were stimulated at 37°C for 2 minutes with AYPGKF (500 μM). Equal amounts of proteins from membrane fractions were analyzed for Akt translocation by western blot analysis using anti-Akt rabbit polyAb (Cell Signaling Technology). (C) Washed human platelets were stimulated with 2MeSADP (100 nM) in the presence or absence of 50 nM YM254890. The effect on Akt translocation and phosphorylation (Ser473 and Thr308) was evaluated by using western blot analysis of platelet membrane fractions using anti-Akt mouse mAb (Santa Cruz Biotechnologies) in panels A and C, and anti-Akt rabbit polyAb (Cell Signaling Technology) in panel B. The western blot analysis shown is representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/1/10.1182_blood-2014-05-576306/4/m_175f3.jpeg?Expires=1768127042&Signature=QErGtbhLp1k8sgdYxmJUYJL8fTWq9exWy8OeuEzuJudRwmhvo9wi57uZXgWAKfF6-idFskRsgon~pJF8TpDxBNGW8~j6C6Lo-h9IQELDS16LJ17xs8dfStazCsfboRW8HU0~VBO3cJRFdD3EnadTVhrzL2U-Lq~yzYQYb99c56Rpra4w2TuqGV0CxHMHpyq23WeRKRnPBDozqRLcnN0O6Lu1Sq4nqgEV0xVIM8NMELkZfLKZ3xVo5P2c1KRMkDLCBAbtlqR~wAsJqzNGGRKRgWM3HgPfU066Ejo9860K93eq-Hjk90BjEqeZqfVPvfoalaF~a1OTLOIrD-pdp9jh3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Effect of PI3K activity/Gi pathway on Akt translocation in human and murine platelets. Washed human platelets were stimulated with AYPGKF (500 μM) (A), SFLLRN (5 μM) (B), or 2MeSADP (100 nM) (C) in the absence or presence of LY294002 (25 μM; a pan-PI3K inhibitor). (D) Washed human platelets were stimulated with AYPGKF (500 μM) in presence or absence of ARC69931MX (100 nM; a P2Y12 antagonist). The effects of inhibitor/antagonist on Akt translocation and phosphorylation were determined by western blot analysis. The data shown are representative of 3 experiments. (E) WT and P2Y12-deficient (knockout [KO]) murine platelets were stimulated at 37°C for 2 minutes with AYPGKF (500 μM). Equal amounts of proteins from membrane fractions were analyzed for Akt translocation by western blot analysis. The western blot analysis shown is representative of 3 independent experiments using anti-Akt rabbit polyAb (Cell Signaling Technology) in panels A and E, and anti-Akt mouse mAb (Santa Cruz Biotechnologies) in panels B-D.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/1/10.1182_blood-2014-05-576306/4/m_175f4.jpeg?Expires=1768127042&Signature=cw1TfJj8bNTkG3G3KDVYrKqi0JeDKBOycQUp7vwiPBLA1cyCfK8-kk1izyEhEUTpk8M2~YEx~Np53wUtztK0z6eCZ0LD11~4yetNKHJVzIjJ3veDkcgHWG7OjQ~Lxw9FBDFQKlUNWk9hdNZ2-vuCrOGRnPj7mtnaKszOiM7s-f~hVJiifQIsSq5LeNC0ClUf9ukWis1974TwRf5FGO2lzTvTE~LPHGGnj2OgD5nV3EJJ16vmHc0B4Jt2rnT-VZuS9iz-GbRBP1yI1mDOg5zdfDVGE3iUGUgccGb6-aaAGm7QB4MEnZzwBUcNO92Vc8L9RUqeahQPw3zm9xFeh7HpHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



