In this issue of Blood, Hu et al identify a critical role for endogenous miR-29a in hematopoietic stem cell (HSC) homeostasis, including the regulation of HSC proliferation and survival.1
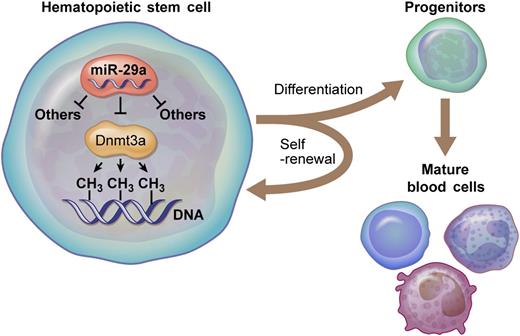
miR-29a targets Dnmt3a, a DNA methyltransferase, in HSCs. These molecular interactions are critical for normal HSC functions, including properly regulated self-renewal and differentiation. Professional illustration by Ken Probst, XavierStudio.
miR-29a targets Dnmt3a, a DNA methyltransferase, in HSCs. These molecular interactions are critical for normal HSC functions, including properly regulated self-renewal and differentiation. Professional illustration by Ken Probst, XavierStudio.
HSCs must strike a careful balance between self-renewal and differentiation into hematopoietic progenitor populations, and the molecular mechanisms that control these events are still being elucidated. There is emerging evidence that microRNAs (miRNAs) might be well suited to regulate these decisions due to their ability to modulate gene expression networks and confer robustness to developmental systems. Consistent with this, recent work has found that a subset of mammalian miRNAs, including miR-29a,2 miR-126,3 miR-155,4 and miR-125a/b,4,5 are expressed in HSCs and sufficient to alter HSC biology following their overexpression or inhibition in vivo. In some cases, including miR-29a2 and miR-125a/b,4 enforced expression triggered myeloproliferative disorders and eventual leukemic transformation in mice, indicating that these miRNAs could also be functionally relevant in human acute myeloid leukemia (AML).
Although overexpression of some HSC miRNAs results in hematopoietic engraftment phenotypes during competitive reconstitution experiments, there is little genetic evidence to date demonstrating that specific endogenous miRNAs are important for normal HSC function beyond a recent study that found an HSC protective role for miR-146a during chronic inflammation.6 However, as hematopoietic-specific deletion of Dicer (which is required for miRNA biogenesis) results in impaired stem cell engraftment and hematopoietic reconstitution,5 there is a fundamental need to determine which endogenously expressed miRNAs promote stem cell functions during steady-state conditions.
In the current study, Hu et al1 find that mice lacking miR-29a/b-1 bicistron have reduced HSC numbers correlating with enhanced HSC proliferation and cell death and reduced multilineage engraftment following competitive bone marrow reconstitution (see figure). Although this mouse strain is deficient in both miR-29-a and miR-29-b1, only expression of miR-29a was able to rescue this hematopoietic phenotype. Providing mechanistic insight, transcriptional profiling of miR-29a/b-1–deficient HSCs uncovered a battery of miR-29 direct targets with relevance to stem cell biology, including Dnmt3a. Importantly, several miR-29a/b1 phenotypes could be partially rescued by crossing miR-29a/b-1−/− mice with Dnmt3a+/− mice to specifically reduce Dnmt3a levels in HSCs lacking miR-29a/b-1. This provides genetic evidence that this particular DNA methyltransferase, a known regulator of HSCs,7 is a functionally relevant target of miR-29a in this context.
In addition to expanding our understanding of how miR-29a has evolved to modulate HSC biology under physiological circumstances, the connection between miR-29a and Dnmt3a also provides important mechanistic insight into how miR-29a might epigenetically influence AML phenotypes. Dnmt3a acquires loss-of-function somatic mutations that result in reduced DNA methylation in a high percentage of AML patients with intermediate-risk cytogenetic profiles or FLT3 mutations, and is associated with a poor prognosis.8 As miR-29a directly targets Dnmt3a,1,9 this may prove to be an important step during the oncogenic process in certain types of AML.
With the identification of miRNAs that drive malignant phenotypes and recent progress in developing various methods to target specific miRNAs therapeutically, miRNAs such as miR-29a may one day be an effective target to treat AML. However, the decision to pursue miR-29a targeting as a means to mitigate AML might prove to be complicated, as a seemingly paradoxical role for exogenous miR-29 family members in blocking AML cell line growth has also been reported.10 This implies that the miR-29 family may play varying roles in AML depending upon the cellular context and possibly the stage of disease where its expression becomes perturbed. Thus, further work is needed to unravel these complexities to both better understand its biology and enable intelligent targeting approaches in the clinic.
The discovery that endogenous miR-29a plays an indispensable role in HSCs through a mechanism involving repression of Dnmt3 is an important step toward defining the molecular circuitry underlying HSC biology. It also justifies additional genetic studies to continue exploring other candidate miRNAs that may have imperative functions in this vital cellular compartment.
Conflict-of-interest disclosure: The author declares no competing financial interests.