Key Points
miR-29a maintains HSC function by targeting Dnmt3a.
Abstract
Hematopoietic stem cells (HSCs) possess the ability to generate all hematopoietic cell types and to self-renew over long periods, but the mechanisms that regulate their unique properties are incompletely understood. Herein, we show that homozygous deletion of the miR-29a/b-1 bicistron results in decreased numbers of hematopoietic stem and progenitor cells (HSPCs), decreased HSC self-renewal, and increased HSC cell cycling and apoptosis. The HSPC phenotype is specifically due to loss of miR-29a, because miR-29b expression is unaltered in miR-29a/b-1-null HSCs, and only ectopic expression of miR-29a restores HSPC function both in vitro and in vivo. HSCs lacking miR-29a/b-1 exhibit widespread transcriptional dysregulation and adopt gene expression patterns similar to normal committed progenitors. A number of predicted miR-29 target genes, including Dnmt3a, are significantly upregulated in miR-29a/b-1-null HSCs. The loss of negative regulation of Dnmt3a by miR-29a is a major contributor to the miR-29a/b-1-null HSPC phenotype, as both in vitro Dnmt3a short hairpin RNA knockdown assays and a genetic haploinsufficiency model of Dnmt3a restored the frequency and long-term reconstitution capacity of HSCs from miR-29a/b-1-deficient mice. Overall, these data demonstrate that miR-29a is critical for maintaining HSC function through its negative regulation of Dnmt3a.
Introduction
Hematopoietic stem cells (HSCs) are the only cells in the blood system capable of giving rise to all mature hematopoietic cells while self-renewing for the lifetime of an organism.1,2 Although HSCs have been extensively characterized with respect to expression of their protein-encoding transcripts,3,4 relatively little is known about the role of non–protein-encoding RNAs in HSC function, particularly microRNAs (miRNAs). miRNAs are small noncoding RNAs (22-24 nts) that exert their biological effects by negatively regulating the stability and/or translation efficiency of multiple target mRNAs.5 Given the ability of each miRNA to target hundreds of mRNAs on average, it is not surprising that miRNAs play critical roles in regulating gene networks involved in stem cell self-renewal, development, and carcinogenesis.6,7
Our laboratory and others have performed miRNA expression profiling studies using highly purified HSCs and committed progenitors in both the mouse and human hematopoietic systems and demonstrated that different hematopoietic stem and progenitor cell (HSPC) populations exhibit unique miRNA expression patterns, supporting a role for miRNAs in their distinctive biological functions.8-11 For example, miRNAs expressed at high levels in HSCs such as miR-125a/b,12-14 miR-126,15 and miR-146a16 regulate HSC self-renewal or differentiation. More recently, miR-29a was shown to play an important role in directly regulating innate and adaptive immune responses17 by targeting interferon-γ18 or indirectly by protecting the thymus from inappropriate involution through suppression of the interferon-α receptor.19 The miR-29 family is also more broadly relevant to stem cell biology, as miR-29b recently was shown to be upregulated by Sox2 and required for reprogramming of fibroblasts into induced pluripotent stem cells.20 Although little is known about how miR-29a transcript levels are regulated, CCAAT/enhancer-binding protein alpha (CEBPA) has been reported to positively regulate the miR-29a/b-1 cluster and expression of miR-29a/b is suppressed in acute myeloid leukemia (AML) patients with impaired CEBPA function.21
To understand the physiologic role of miR-29a in normal hematopoietic development, we evaluated HSPCs in mice harboring a genetic deletion of the miR-29a/b-1 bicistron.19 Here we demonstrate that deletion of the miR-29a/b-1 bicistron results in decreased HSC self-renewal and long-term reconstitution capacity. This loss of HSC function is associated with increased HSC cell cycling and apoptosis, as well as acquisition of a gene expression profile similar to more differentiated hematopoietic progenitors. Among the differentially expressed transcripts are multiple predicted miR-29a targets including Dnmt3a. By crossing miR-29a/b-1 heterozygous mice to Dnmt3a heterozygous mice, we show that the functional defects observed in miR-29a-null HSCs are largely mediated via abrogation of its negative regulation of Dnmt3a expression. Overall, these studies indicate that miR-29a is essential in maintaining HSC function and mediates its effects by modulating the activity of the epigenetic regulator Dnmt3a.
Materials and methods
Retrovirus preparation and transduction
The Dnmt3a short hairpin RNA (shRNA) constructs were cloned into the pMig plasmid and were gifts from Dr Iannis Aifantis (New York University, New York, NY). Retroviral preparation and donor cell infections/transplantations were performed as previously described.9 The ability of 2 shRNA clones (197 and 6567) to knock down Dnmt3a was confirmed in NIH/3T3 cells. DNMT3a antibody (2160S; Cell Signaling) was used to confirm the protein levels after knockdown.
Mice/transplantations
The generation of miR-29a/b-1-null mice was previously described.19 Donor and recipient mice (C57BL/6 and B6.SJL-Ptprca Pepcb/BoyJ, respectively) were bred and maintained in the Memorial Sloan Kettering Cancer Center mouse facility. Dnmt3awt/fl[Mx-Cre] mice were crossed with miR-29a/b-1 Het mice, and their progeny were injected intraperitoneally 6 times with 300 μg polyinosinic:polycytidylic acid (Sigma) in phosphate-buffered saline every other day to induce deletion of miR-29a/b-1 floxed alleles. All progeny contained Mx-Cre knockin to diminish the bias from Cre expression. Recipients were retro-orbitally transplanted following lethal irradiation using a γ radiation source (9.5 Gy total) and maintained on antibiotics (Sulfatrim) for 6 weeks following transplantation. Total bone marrow cells (2 million donor cells) or magnetic bead-enriched (Miltenyi Biotec) c-Kit+ cells (500 000 cells) were used for noncompetitive or competitive transplants, respectively. Competitive transplantations were performed with equal numbers of competitor bone marrow cells. Following transplant, the peripheral blood was sampled monthly to evaluate donor chimerism and lineage composition. All mouse procedures were performed in accordance with institutional guidelines as described in an Institutional Animal Care and Use Committee (IACUC) approved protocol.
miRNA expression analysis
The expression of miR-29a, miR-29b, and miR-29c was measured using a QuantiMir kit per the manufacturer’s instructions (System Biosciences). Synthesized oligonucleotides containing mature miRNA sequences were used as primers for miR-29 family member genes. Total RNA was prepared from total or c-Kit+-enriched bone marrow cells using the RNeasy Mini Kit (Qiagen). Mouse snoRNA202 was used as an endogenous control to normalize for total RNA loaded.
Methylcellulose colony forming assays
To evaluate self-renewal and proliferation of HSPCs, fluorescence-activated cell sorter (FACS)-purified c-Kit+ HSPCs were cultured in methylcellulose medium supplemented with cytokines (Methocult GF M3434; Stem Cell Technologies). Colony numbers were counted 12 days after plating. Serial replating cultures were performed by harvesting cells from methylcellulose media, followed by plating 20 000 cells in fresh methylcellulose.
Cell staining and flow cytometry
Mouse bone marrow cells were harvested and stained as previously described.22 Briefly, antibodies used in this study include a lineage (Lin) cocktail containing antibodies against Ter-119 (clone Ter-119), B220 (RA3-6B2), CD3e (145-2C11), CD4 (GK1.5), CD8 (53-6.7), Gr-1 (RB6-8C5), and Mac-1 (M1/70) antibodies, conjugated with either phycoerythrin (PE)-Cy5 or PE-Cy7 (eBiosciences). Additional antibodies used to identify HSPCs included CD16/32 (93) in Alexa eFluor 700, IL7Ra (A7R34) in PE-Cy5, CD45.1 (A20) in PE-Cy7, c-Kit (2B8) in allophycocyanin (APC)-eFluor 780, Gr-1 (RB6-8C5) in PE, CD45.2 (104) in Alexa eFluor 700, CD34 (RAM34) in fluorescein isothiocyanate, Flk2/Flt3 (A2F10) in PE (all from eBiosciences), as well as Sca-1 (E13-161.7) in Pacific Blue and CD150 (TC15-12F12.2) in PE (both from BioLegend). After staining, cells were analyzed and sorted using a FACSAria II (Becton Dickinson). All cell populations were double sorted, and a purity of >90% was routinely achieved. Flow cytometry data were analyzed using Flowjo software (TreeStar, Inc.).
Cell cycle and apoptosis analysis
For cell cycle analysis, bone marrow cells were stained with antibodies to identify HSPCs as described above and then fixed using a Fixation/Permeabilization Kit (eBioscience) and stained with 4,6 diamidino-2-phenylindole (DAPI; Invitrogen). 5-Bromo-2′-deoxyuridine (BrdU) retention assays were performed by analyzing bone marrow cells 20 hours after intraperitoneal injection with 150 μL BrdU (10 mg/mL) per mouse (BD Pharmingen). A fluorescein isothiocyanate-conjugated anti-BrdU antibody was used to detect BrdU content by flow cytometry. Apoptosis was evaluated using an Annexin V staining kit per the manufacturer’s instructions (BD).
Microarray analysis
Total RNA was extracted from double FACS-sorted cell populations using an RNeasy Mini Kit (Qiagen), Nugen Ovation Pico WTA was used for preamplification of RNA samples and was hybridized to Illumina Mouse 6 arrays using standard protocols. Array data were analyzed using open source R/Bioconduct software including lumi and limma packages (R Development Core Team, 2012). The microarray data were submitted to the Gene Expression Omnibus (GEO) database (accession number GSE58237). Predicted miR-29a/b-1 target genes were identified using the online database targetscan.org.23
Data analysis and statistics
Data were summarized and presented using open source R/Bioconductor software (R Development Core Team, 2012). Statistical tests were performed using the appropriate software packages accompanying this program, including a 2-tailed t test between groups (*P < .05; **P < .01); log-rank tests were used for comparison of Kaplan-Meier curves.24 Error bars in bar graphs indicate standard error of the mean (SEM).
Results
miR-29a/b-1 positively regulates hematopoietic stem/progenitor cell number
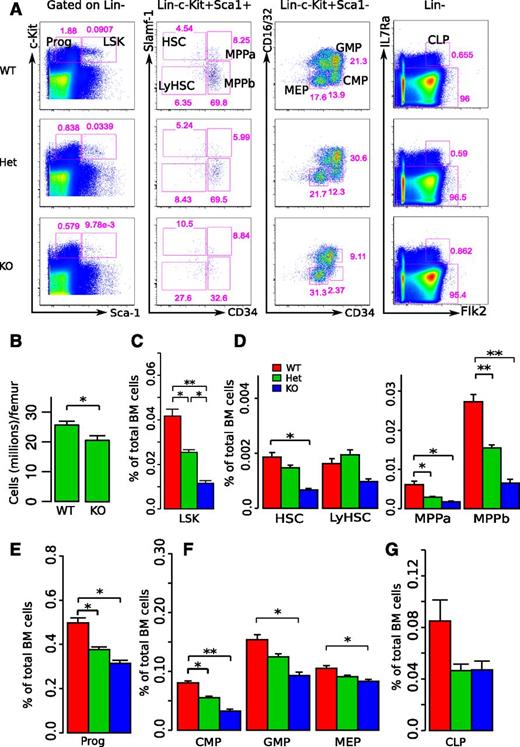
To determine the role of miR-29a/b-1 in hematopoiesis, we assessed the frequencies of HSPCs in mice harboring a constitutive deletion of the miR-29a/b-1 bicistronic cluster on chromosome 6 by flow cytometry (FC).19 In adult mice (12-16 weeks old), deletion of miR-29a/b-1 resulted in significantly decreased frequencies of Lin−c-Kit+Sca-1+ (LSK) and Lin−c-Kit+Sca-1− (committed progenitors) cells compared with wild-type (WT) littermates (Figure 1A,C,E). miR-29a/b-1−/− mice also exhibited a 20.1% decrease in bone marrow cellularity (Figure 1B). Furthermore, deletion of miR-29a/b-1 resulted in significantly reduced frequencies of populations within the LSK population (HSCs and multipotent progenitors [MPPs]), as well as in committed progenitors (common myeloid progenitor [CMP], granulocyte-macrophage progenitor [GMP], megakaryocyte-erythroid progenitor [MEP], and common lymphoid progenitor [CLP]; Figure 1A,D,F-G). Despite these alterations in HSPC composition, miR-29a/b-1−/− mice did not exhibit statistically significant differences in their peripheral blood counts compared with those of their WT littermates (supplemental Figure 1, available on the Blood Web site). In addition, FC analysis of the peripheral blood failed to demonstrate differences in the frequencies of myeloid or T cells in miR-29a/b-1−/− mice compared with WT littermate controls, although miR-29a/b-1−/− mice did demonstrate a significant decrease in B220+ B cells (supplemental Figure 2). To determine whether alterations in HSPC composition are specific to adult hematopoiesis, we evaluated fetal liver hematopoiesis in E13.5∼E14.5 miR-29a/b-1−/− embryos. There was no difference in HSPC frequencies or total cellularity in fetal liver, indicating a developmental stage-specific effect of miR-29a/b-1 function (supplemental Figure 3A-D). These data indicate that loss of miR-29a/b-1 results in a significant reduction in the number of HSPCs during steady-state adult hematopoiesis.
miR-29a/b-1-null mice exhibit decreased bone marrow cellularity and significantly reduced numbers of HSPCs. (A) Representative flow cytometric analyses. Each column includes WT littermate, miR-29a/b-1 Het, and miR-29a/b-1-null (KO) mice. Live cells were gated based on propidium iodide exclusion. Prior gates for each subpopulation are indicated on the top of each column. Specific HSPC populations and their frequencies were calculated based on total bone marrow cell counts and were identified as shown. (B) Total bone marrow cell counts were based on bilateral femur cell recoveries. (C-G) Summarized results of bone marrow HSPC frequencies assessed by flow cytometry. The cell populations evaluated were indicated at the x-axis, and the y-axis represents the frequencies of these populations among total bone marrow cells. Each group included 7 to 10 mice. Error bars indicated SEM. Statistical significance was calculated using a Student t test: *P < .05; **P < .01.
miR-29a/b-1-null mice exhibit decreased bone marrow cellularity and significantly reduced numbers of HSPCs. (A) Representative flow cytometric analyses. Each column includes WT littermate, miR-29a/b-1 Het, and miR-29a/b-1-null (KO) mice. Live cells were gated based on propidium iodide exclusion. Prior gates for each subpopulation are indicated on the top of each column. Specific HSPC populations and their frequencies were calculated based on total bone marrow cell counts and were identified as shown. (B) Total bone marrow cell counts were based on bilateral femur cell recoveries. (C-G) Summarized results of bone marrow HSPC frequencies assessed by flow cytometry. The cell populations evaluated were indicated at the x-axis, and the y-axis represents the frequencies of these populations among total bone marrow cells. Each group included 7 to 10 mice. Error bars indicated SEM. Statistical significance was calculated using a Student t test: *P < .05; **P < .01.
Loss of miR-29a/b-1 results in impaired stem/progenitor cell colony forming capability
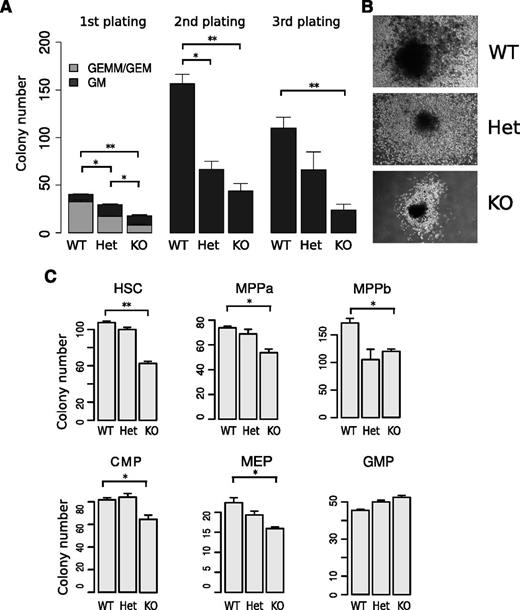
To determine whether the reduction in HSPC numbers in miR-29a/b-1-deficient mice is due to decreased HSPC self-renewal and/or proliferative capacity, we performed methylcellulose colony formation assays with serial replating. miR-29a/b-1−/− cells gave rise to fewer and smaller colonies compared with WT littermate controls when 3000 c-Kit+ cells were plated into methylcellulose media (Figure 2A-B). The reduction in colony forming activity in miR-29a/b-1−/− cells compared with WT controls was more severe in secondary plating experiments, and by the third plating, too few cells were generated from miR-29a/b-1−/− cells to be replated (Figure 2A).
miR-29a/b-1-null HSPCs exhibit decreased colony forming capacity in methylcellulose colony assays. (A) Methylcellulose colony assays were performed using c-Kit+-enriched bone marrow cells. The first plating was initiated using 3000 c-Kit+ cells from WT, Het, and KO mice. Replatings were performed using 20 000 cells per well. (B) Photomicrographs of typical colonies generated by WT, Het, and KO HSPCs on initial plating. (C) Methylcellulose colony assays were performed using purified HSPCs. Six populations, including HSC, MPPa, MPPb, CMP, MEP, and GMP, were double FACS sorted and 150 (HSC, MPPa, MPPb) or 400 (CMP, MEP, and GMP) cells were plated. Data shown represent the aggregate of 3 technical replicates. Error bars indicate SEM. Statistical significance was calculated using a Student t test: *P < .05; **P < .01.
miR-29a/b-1-null HSPCs exhibit decreased colony forming capacity in methylcellulose colony assays. (A) Methylcellulose colony assays were performed using c-Kit+-enriched bone marrow cells. The first plating was initiated using 3000 c-Kit+ cells from WT, Het, and KO mice. Replatings were performed using 20 000 cells per well. (B) Photomicrographs of typical colonies generated by WT, Het, and KO HSPCs on initial plating. (C) Methylcellulose colony assays were performed using purified HSPCs. Six populations, including HSC, MPPa, MPPb, CMP, MEP, and GMP, were double FACS sorted and 150 (HSC, MPPa, MPPb) or 400 (CMP, MEP, and GMP) cells were plated. Data shown represent the aggregate of 3 technical replicates. Error bars indicate SEM. Statistical significance was calculated using a Student t test: *P < .05; **P < .01.
To investigate the effect of miR-29a/b-1 loss on specific HSPC populations, we performed colony formation assays using FACS-sorted HSC, MPP, CMP, GMP, and MEP cells.25-28 All these miR-29a/b-1-deficient populations exhibited significant reductions in colony numbers with the exception of GMPs (Figure 2C), but all HSPC populations, including GMPs, gave rise to smaller colonies. The differences in colony formation were more pronounced in cultures initiated by the most immature populations (ie, HSCs and MPPs; Figure 2C). Together, these data indicate that miR-29a/b-1 positively regulates HSPC proliferation and self-renewal capacity.
miR-29a/b-1-null HSCs exhibit cell-intrinsic defects in self-renewal in vivo
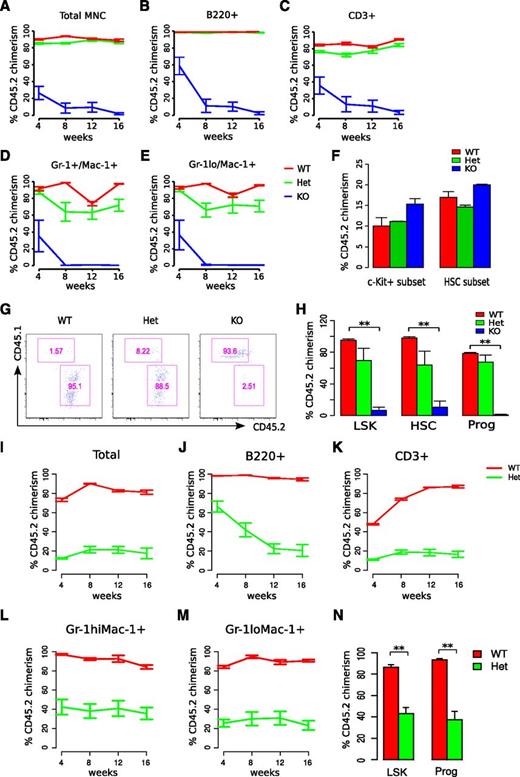
To further examine the self-renewal defect of miR-29a/b-1-null HSCs, as well as to determine whether the hematologic phenotype is cell intrinsic, long-term reconstitution assays were performed using total bone marrow cells, as well as partially purified HSPC populations. Lethally irradiated mice (CD45.1, B6.SJL-Ptprca Pepcb/BoyJ) were transplanted with 2 million unfractionated, CD45.2-positive bone marrow cells from miR-29a/b-1-null (KO), miR-29a/b-1+/− (Het), or WT littermate controls. Donor chimerism was monitored serially in transplanted recipients by sampling the peripheral blood. Although WT and Het bone marrow cells gave rise to lymphoid and myeloid progeny at similar frequencies (92.3% and 85.6% donor chimerism, respectively, at week 4 after transplantation), KO bone marrow cells reconstituted transplanted recipients poorly (28.5% donor chimerism at week 4), and their chimerism levels decreased progressively over time (Figure 3A-E).
miR-29a/b-1-deficient HSCs exhibit reduced self-renewal and reconstitution capacity. (A-E) Donor cell chimerism of total peripheral blood leukocytes or specific cell lineages in the peripheral blood was examined following total bone marrow cell transplant. Donor chimerism (CD45.2+) levels were evaluated following transplantation of 2 million total bone marrow cells from WT, Het, or KO mice into lethally irradiated recipients. (F) Bone marrow cells from both femurs and tibias were examined for donor chimerism 20 hours after transplant using the same transplantation protocol used for competitive transplants. Each group includes 6 to 10 recipient mice transplanted with cells from >3 different donors. (G) Representative flow cytometric analysis of HSPCs in WT, Het, and KO recipients 16 weeks after transplant, previously gated on LSK cells. (H) Summarized results of flow cytometric analysis of bone marrow cells in recipients 4 months after the second transplantation. (I-M) Donor cell chimerism of secondary recipients transplanted with total bone marrow cells from primary recipients transplanted with WT or Het cells. (N) Flow cytometric analysis of HSPCs in the bone marrow of secondary recipients. Statistical significance was calculated using a Student t test: *P < .05; **P < .01.
miR-29a/b-1-deficient HSCs exhibit reduced self-renewal and reconstitution capacity. (A-E) Donor cell chimerism of total peripheral blood leukocytes or specific cell lineages in the peripheral blood was examined following total bone marrow cell transplant. Donor chimerism (CD45.2+) levels were evaluated following transplantation of 2 million total bone marrow cells from WT, Het, or KO mice into lethally irradiated recipients. (F) Bone marrow cells from both femurs and tibias were examined for donor chimerism 20 hours after transplant using the same transplantation protocol used for competitive transplants. Each group includes 6 to 10 recipient mice transplanted with cells from >3 different donors. (G) Representative flow cytometric analysis of HSPCs in WT, Het, and KO recipients 16 weeks after transplant, previously gated on LSK cells. (H) Summarized results of flow cytometric analysis of bone marrow cells in recipients 4 months after the second transplantation. (I-M) Donor cell chimerism of secondary recipients transplanted with total bone marrow cells from primary recipients transplanted with WT or Het cells. (N) Flow cytometric analysis of HSPCs in the bone marrow of secondary recipients. Statistical significance was calculated using a Student t test: *P < .05; **P < .01.
One possible explanation for the decreased engraftment capacity of KO HSCs is that they possess intrinsic defects in their bone marrow homing capacity. To examine this possibility, we competitively transplanted 500 000 c-Kit+ cells from WT, Het, and KO mice (CD45.2) into lethally irradiated congenic recipients (CD45.1), along with 500 000 total bone marrow cells expressing the recipient CD45.1 allele. After 20 hours, mice were euthanized, and bone marrow cells were evaluated for the presence of donor cells. Equal numbers of KO, Het, and WT donor HSCs (Lin−Sca-1+c-Kit+Slamf1+) were present in the bone marrows of recipients, thereby providing no support for a homing defect (Figure 3F).
Analysis of long-term engrafted mice revealed that transplanted KO cells contributed to HSPCs at lower levels than WT littermate controls (Figure 3G-H). Similar deficits in HSPC engraftment and reconstitution were observed when 500 000 c-Kit+ bone marrow cells from KO mice were competitively transplanted with 500 000 WT total bone marrow cells (supplemental Figure 4A-E). Het donor chimerism in the peripheral blood was also significantly lower than WT littermates when transplantations were performed competitively. These data indicate that miR-29a/b-1 regulates HSC long-term reconstitution capacity.
To further characterize the defects in miR-29a/b-1 Het HSPC function, we transplanted total bone marrow cells from noncompetitively engrafted recipients exhibiting high levels of WT or Het donor chimerism (∼90%). Secondary transplantation of Het bone marrow cells gave rise to significantly lower levels of CD45.2 donor chimerism than WT cells (Het 11.5% vs WT 72.3% at 4 weeks; P < .05), with Het donor chimerism levels remaining low over time (Figure 3I-M). Both LSK and committed progenitor cells from Het HSPC transplanted recipients were present in the BM of secondary recipients at significantly lower frequencies at 16 weeks after transplantation compared with recipients receiving WT cells (Figure 3N), consistent with a gene dosage-dependent role of miR-29a/b-1 in sustaining HSC self-renewal.
miR-29a/b-1 maintains HSC quiescence and promotes cell survival
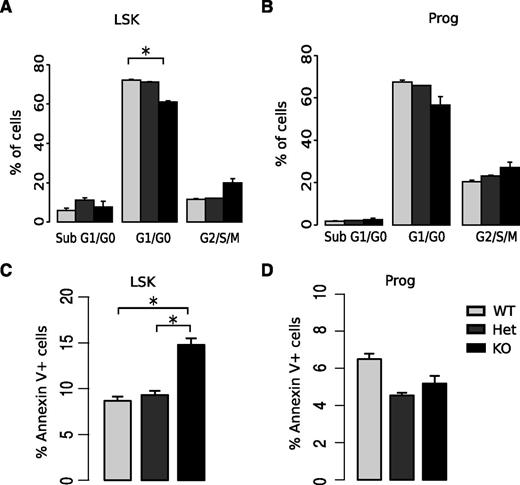
Because HSC quiescence is highly associated with self-renewal capacity,29 we evaluated the cell cycle status of KO HSPCs. Although KO LSK cells exhibited a significantly higher percentage of cells in the S-G2-M phases of the cell cycle compared with WT littermate LSK cells (Figure 4A; supplemental Figure 5), KO committed progenitors did not exhibit alterations in cell cycle distribution (Figure 4B). To confirm that these findings reflected differences in cell cycle progression, we assessed cell cycle entry using BrdU incorporation assays and found that LSK cells from KO mice cycled more rapidly than WT littermate controls (41.63% vs 58.64% in G0, respectively; P < .05; supplemental Figure 6). As decreased HSC number and reduced bone marrow cellularity may be due to increased apoptosis, we performed annexin V staining. KO mice exhibited a higher frequency of apoptotic LSK cells (annexin V+7-aminoactinomycin D (7-AAD)−) than WT littermates. In contrast, there was no difference in apoptosis observed in committed progenitors (Figure 4C-D; supplemental Figure 7). Together, these results demonstrate that miR-29a/b-1 loss impairs the maintenance of quiescence and cell survival specifically in LSK cells, which are composed of the most immature hematopoietic cells including HSCs and MPPs.
Deletion of miR-29a/b-1 is associated with increased HSPC cell cycling and apoptosis. (A-B) Cell cycle analysis by staining DNA content of fixed cells with DAPI. Fewer LSK cells, but not committed progenitor cells, accumulated in the G1/G0 stages of the cell cycle in miR-29a KO mice. (C-D) Increased numbers of annexin V+ LSK cells, but not myeloid progenitor cells, were present in both miR-29a KO and Het mice compared with WT. Details of annexin V staining and cell cycle staining for specific HSPC populations are summarized in supplemental Figures 5 and 7. Error bars indicate SEM. Each group has 7 to 10 mice from different experiments. Statistical significance was calculated using a Student t test: *P < .05; **P < 0.01.
Deletion of miR-29a/b-1 is associated with increased HSPC cell cycling and apoptosis. (A-B) Cell cycle analysis by staining DNA content of fixed cells with DAPI. Fewer LSK cells, but not committed progenitor cells, accumulated in the G1/G0 stages of the cell cycle in miR-29a KO mice. (C-D) Increased numbers of annexin V+ LSK cells, but not myeloid progenitor cells, were present in both miR-29a KO and Het mice compared with WT. Details of annexin V staining and cell cycle staining for specific HSPC populations are summarized in supplemental Figures 5 and 7. Error bars indicate SEM. Each group has 7 to 10 mice from different experiments. Statistical significance was calculated using a Student t test: *P < .05; **P < 0.01.
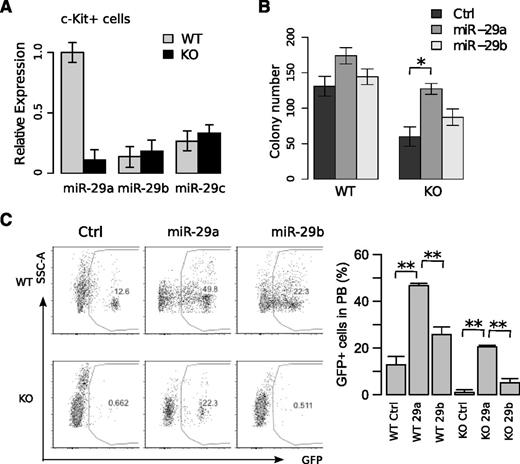
miR-29a/b-1-null hematopoietic phenotype is due to miR-29a loss
As the miR-29a/b-1 KO mouse harbors a deletion of 2 miRNAs, and the miR-29 family is represented by 2 bicistrons including miR-29a/miR-29b-1 on chromosome 7 and miR-29b-2/miR-29c on chromosome 1, we sought to determine which miR-29 family member is responsible for the miR-29a/b-1 KO HSPC phenotype. By quantitative real-time polymerase chain reaction (qRT-PCR), we confirmed that miR-29a expression was absent in c-Kit+ cells from KO mice, whereas miR-29b and miR-29c expression were not significantly altered (Figure 5A). Using retroviruses expressing miR-29a or miR-29b, only ectopic overexpression of miR-29a in KO LSKs significantly increased colony numbers (Figure 5B). Finally, the ability of miR-29a to reconstitute the function of KO LSK cells was confirmed in transplantation assays in which overexpression of miR-29a, but not miR-29b, restored KO donor cell chimerism in a multilineage fashion (Figure 5C; data not shown). Together, these data indicate that miR-29a is responsible for the functional defects observed in the KO mice, which we now refer to miR-29a-deficient mice.
Overexpression of miR-29a, but not miR-29b, rescues the self-renewal defect in miR-29a/b-1-null HSCs. Transcript levels of miR-29 members were measured in (A) c-Kit-enriched cells. (B) Methylcellulose colony forming capability of LSK cells after retroviral overexpression of miR-29a or miR-29b in miR-29a/b-1-null HSPCs. Twenty-four hours following transduction, GFP+ cells were sorted, and 300 were plated per well. Each group represents experiments using 2 mice, with wells plated in triplicate. (C) Transplantation of 300 000 LSK cells from WT or miR-29a/b-1-null HSPCs retrovirally transduced with miR-29a, -29b, or GFP control (n = 6-10 mice per group). Peripheral blood analysis was performed 16 weeks after transplantation. Data represent the aggregate of 2 independent experiments. Error bars indicate SEM. Statistical significance was calculated using a Student t test: *P < .05.
Overexpression of miR-29a, but not miR-29b, rescues the self-renewal defect in miR-29a/b-1-null HSCs. Transcript levels of miR-29 members were measured in (A) c-Kit-enriched cells. (B) Methylcellulose colony forming capability of LSK cells after retroviral overexpression of miR-29a or miR-29b in miR-29a/b-1-null HSPCs. Twenty-four hours following transduction, GFP+ cells were sorted, and 300 were plated per well. Each group represents experiments using 2 mice, with wells plated in triplicate. (C) Transplantation of 300 000 LSK cells from WT or miR-29a/b-1-null HSPCs retrovirally transduced with miR-29a, -29b, or GFP control (n = 6-10 mice per group). Peripheral blood analysis was performed 16 weeks after transplantation. Data represent the aggregate of 2 independent experiments. Error bars indicate SEM. Statistical significance was calculated using a Student t test: *P < .05.
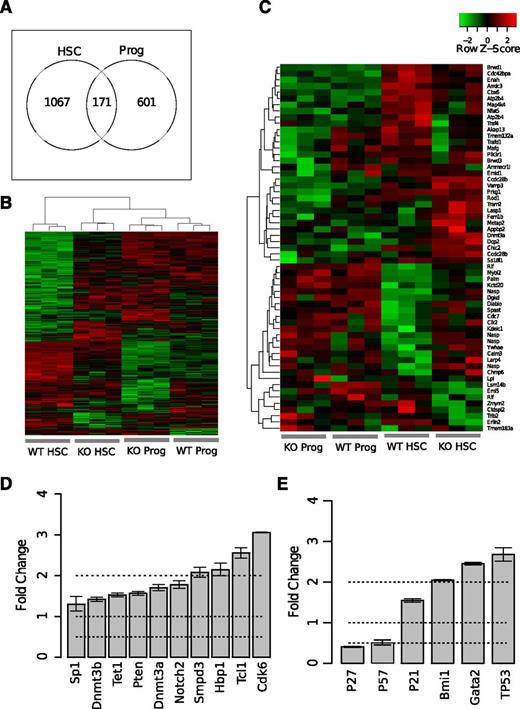
miR-29a promotes HSC differentiation by regulating multiple target genes
In silico algorithms predict that miR-29a targets several hundred mRNAs (eg, targetscan.org).7 However, the interactions between miRNAs and their target genes depend on their relative expression levels.30 To better understand the molecular pathways altered due to loss of miR-29a in HSCs, we performed gene expression profiling of FACS-purified HSCs (Lin−c-Kit+Sca-1+CD34−Slamf1+) and committed progenitors (Lin−c-Kit+Sca-1−) from miR-29a KO mice. Using a P value cutoff of <.05, our analysis showed that miR-29a/b-1 homozygous deletion resulted in dysregulated expression of a larger number of genes in HSCs (1238 genes) than in committed progenitor cells (772 genes; Figure 6A). Unsupervised hierarchical clustering analysis revealed that the KO HSCs exhibit an altered gene expression profile compared with WT HSCs, and their expression patterns are more closely related to WT committed progenitors than WT HSCs (Figure 6B). Gene set enrichment analysis31 demonstrated a significant enrichment of cell cycle regulators in KO HSCs, consistent with their altered cell cycle status (supplemental Figure 8). In an unsupervised hierarchical clustering analysis considering predicted miR-29a target genes,7,23 we identified a group of these genes similarly expressed in WT and miR-29a-null committed progenitors, but significantly upregulated in miR-29a-null HSCs compared with WT HSCs. These genes included Dnmt3a, Diablo, Spast, Cdc7, Clk2, Kdelc1, Nasp, Ywhae, Calm3, Larp4, and Chmp6 (Figure 6C). To validate our microarray results, we performed qRT-PCR to measure the expression of dysregulated miR-29a target genes and additional genes that regulate HSC function using c-Kit+ bone marrow cells from the KO and WT littermates. We confirmed the increased expression of predicted miR-29a targets in KO cells, as well as additional genes that regulate HSC function such as p21, p27, Bmi1, Gata2, and TP53 (Figure 6D-E).
Gene expression profiling analysis reveals dysregulation of miR-29a targets in HSCs. (A) Numbers of differentially expressed genes in miR-29a/b-1 KO or WT HSCs (Lin−c-Kit+Sca-1+CD34−Slam+) or committed progenitor (Lin−c-Kit+Sca-1−; Prog) cells. Genes were selected using a P value threshold of <.05 and a fold change >1.5. (B) Unsupervised clustering analysis of dysregulated genes identified in A. (C) Unsupervised clustering analysis of genes predicted to be targets of miR-29a. Predicted targets were identified using targetscan.org (Release 6.1). (D) Validation of changes in the expression levels of reported regulators of bone marrow HSPCs using c-Kit-enriched bone marrow cells from miR-29a/b-1 WT or KO mice by qRT-PCR. Fold change was calculated by comparing KO with WT cells. The genes shown represent predicted miR-29a target genes with fold change >1 in miR-29a/b-1 KOs compared with WT littermates. (E) Selected regulators of HSC function were chosen based on published genetic evidence. Fold change was relative to WT type. Error bars represent SEM from triplicate experiments.
Gene expression profiling analysis reveals dysregulation of miR-29a targets in HSCs. (A) Numbers of differentially expressed genes in miR-29a/b-1 KO or WT HSCs (Lin−c-Kit+Sca-1+CD34−Slam+) or committed progenitor (Lin−c-Kit+Sca-1−; Prog) cells. Genes were selected using a P value threshold of <.05 and a fold change >1.5. (B) Unsupervised clustering analysis of dysregulated genes identified in A. (C) Unsupervised clustering analysis of genes predicted to be targets of miR-29a. Predicted targets were identified using targetscan.org (Release 6.1). (D) Validation of changes in the expression levels of reported regulators of bone marrow HSPCs using c-Kit-enriched bone marrow cells from miR-29a/b-1 WT or KO mice by qRT-PCR. Fold change was calculated by comparing KO with WT cells. The genes shown represent predicted miR-29a target genes with fold change >1 in miR-29a/b-1 KOs compared with WT littermates. (E) Selected regulators of HSC function were chosen based on published genetic evidence. Fold change was relative to WT type. Error bars represent SEM from triplicate experiments.
miR-29a regulates HSC function in part through inhibition of Dnmt3a
Transcriptional profiling revealed that Dnmt3a transcript levels were increased 1.55-fold in miR-29a-null HSCs compared with WT HSCs (P = .03), and this result was confirmed by qRT-PCR (Figure 6D). As miR-29 has been shown to target DNA methyltransferase 3a (Dnmt3a) in the context of cancer,32,33 and Dnmt3a plays a prominent role in maintaining HSC function and lineage commitment34 and is frequently mutated in acute myeloid leukemia,35-37 we investigated whether Dnmt3a is required for the miR-29a-deficient HSPC phenotype. To test whether Dnmt3a deficiency can restore normal function in miR-29a-null HSCs, we knocked down Dnmt3a in LSK cells using 2 retroviral shRNAs (196 and 6567) that had been previously validated in NIH/3T3 cells (Figure 7A; supplemental Figure 9). After Dnmt3a shRNA-transduced LSK cells (GFP+) were double FACS-sorted and plated into methylcellulose media, KO LSK cells demonstrated a significant increase in colony formation capacity compared with vector controls (Figure 7B).
Dnmt3a dysregulation mediates miR-29a/b-1-deficient HSC defects. (A) shRNAs (197 and 6567) efficiently knock down Dnmt3a mRNA levels by qRT-PCR in NIH 3T3 cells. (B) Knock down of Dnmt3a partially restores miR-29a/b-1-null HSPC function as assessed by methylcellulose colony formation assay. Bone marrow cells were harvested from fluorouracil (5-FU) treated mice and infected with either empty GFP+ retrovirus or shRNAs against Dnmt3a. GFP+ cells were double-sorted after 2 rounds of infection and plated in 6-well plates with 200 cells per well, and colony numbers were counted at day 12. (C) The 3- to 4-week-old progeny from miR-29a+/−Dnmt3awt/fl[Mx1-Cre] mice were treated with poly I:C 3 times every other day and were analyzed 1 month following the last injection. LSK (left) cells were significantly increased in miR-29a+/−;Dnmt3+/− mice, but committed progenitors were not affected (right). (D) Evaluation of cell cycle status of LSK cells in miR-29a+/−, Dnmt3a+/−, and miR-29a+/−;Dnmt3a+/− mice using a DAPI DNA stain. Shown with representative flow cytometry analysis (left) and summarized results (right). (E) Five million total bone marrow cells from miR-29a+/+;Dnmt3a+/+, miR-29a+/−;Dnmt3a+/+, miR-29a+/+;Dnmt3a+/−, and miR-29a+/−;Dnmt3a+/− mice were transplanted into lethally irradiated congenic recipients with 5 million competitor bone marrow cells. Peripheral blood donor chimerism was determined 4 months after transplant. (F) Bone marrow HSPCs were analyzed for donor cell chimerism. Error bars represent SEM from 5 to 12 mice for all experiments shown. Statistical significance was calculated using a Student t test: *P < .05; **P < .01.
Dnmt3a dysregulation mediates miR-29a/b-1-deficient HSC defects. (A) shRNAs (197 and 6567) efficiently knock down Dnmt3a mRNA levels by qRT-PCR in NIH 3T3 cells. (B) Knock down of Dnmt3a partially restores miR-29a/b-1-null HSPC function as assessed by methylcellulose colony formation assay. Bone marrow cells were harvested from fluorouracil (5-FU) treated mice and infected with either empty GFP+ retrovirus or shRNAs against Dnmt3a. GFP+ cells were double-sorted after 2 rounds of infection and plated in 6-well plates with 200 cells per well, and colony numbers were counted at day 12. (C) The 3- to 4-week-old progeny from miR-29a+/−Dnmt3awt/fl[Mx1-Cre] mice were treated with poly I:C 3 times every other day and were analyzed 1 month following the last injection. LSK (left) cells were significantly increased in miR-29a+/−;Dnmt3+/− mice, but committed progenitors were not affected (right). (D) Evaluation of cell cycle status of LSK cells in miR-29a+/−, Dnmt3a+/−, and miR-29a+/−;Dnmt3a+/− mice using a DAPI DNA stain. Shown with representative flow cytometry analysis (left) and summarized results (right). (E) Five million total bone marrow cells from miR-29a+/+;Dnmt3a+/+, miR-29a+/−;Dnmt3a+/+, miR-29a+/+;Dnmt3a+/−, and miR-29a+/−;Dnmt3a+/− mice were transplanted into lethally irradiated congenic recipients with 5 million competitor bone marrow cells. Peripheral blood donor chimerism was determined 4 months after transplant. (F) Bone marrow HSPCs were analyzed for donor cell chimerism. Error bars represent SEM from 5 to 12 mice for all experiments shown. Statistical significance was calculated using a Student t test: *P < .05; **P < .01.
To confirm that miR-29a and Dnmt3a functionally interact in vivo, we crossed mice deficient in miR-29a and Dnmt3a and found that LSK cell frequencies were similar in WT and Het Dnmt3a mice, and miR-29a Het LSKs were significantly decreased compared with both WT and Het Dnmt3a mice. Mice heterozygous for both Dnmt3a and miR-29a exhibited significantly higher frequencies of LSK cells than miR-29a Het mice, but committed progenitor cell frequencies were unaltered (Figure 7C). This restoration of miR-29a Het LSK cell number on Dnmt3a loss was associated with increased quiescence, supporting a central role of Dnmt3a in the cell cycle alterations observed in miR-29a KO HSCs (Figure 7D). Dnmt3a mRNA expression was upregulated in miR-29a heterozygous LSK cells, and Dnmt3a mRNA levels were restored to WT levels in mice compound heterozygous for Dnmt3a and miR-29a (supplemental Figure 10A). In contrast, miR-29a expression levels were unaltered in Dnmt3a-deficient LSK cells (supplemental Figure 10B). Together, these data indicate that Dnmt3a expression is regulated by miR-29a, and that de-repression of Dnmt3a expression in the context of miR-29a contributes to the defects observed in miR-29a Het and KO HSCs.
To determine whether Dnmt3a reduction can restore miR-29a-deficient HSC function in vivo, we competitively transplanted 5 million total bone marrow cells from WT littermates, miR-29a+/−, Dnmt3a+/−, and miR-29a+/−;Dnmt3a+/− mice into lethally irradiated congenic recipients each along with 5 million competitors. Peripheral blood donor cell chimerism was significantly higher in mice transplanted with compound miR-29a+/−;Dnmt3a+/− cells compared with those transplanted with miR-29a+/− cells 6 months following transplant (Figure 7E). Mice transplanted with miR-29a Het cells exhibited significantly lower donor chimerism among immature hematopoietic cells, but miR-29a+/−;Dnmt3a+/− HSCs demonstrated competitive long-term reconstitution capacity similar to that observed with WT donors when the recipient’s BM and spleen were evaluated (Figure 7F; supplemental Figure 10C-D). We also evaluated HSC function from mice expressing reduced levels of miR-29a, Dnmt3a, or both by competitive transplantation. Evaluation of the peripheral blood 16 weeks following transplant revealed that deletion of Dnmt3a increased the reconstitution capacity of miR-29a-null HSCs compared with WT HSCs, consistent with findings previously observed,34 and this effect was slightly reduced in the setting of miR-29a heterozygosity. Dnmt3a heterozygosity resulted in a small, but statistically significant, increase in donor cell chimerism in the miR-29a-null background (supplemental Figure 11). Together, these data confirm that Dnmt3a is a critical downstream mediator of miR-29a function in HSCs.
Discussion
In this study, we assessed the role of miR-29a in hematopoiesis by evaluating mice lacking the miR-29a/b-1 bicistron. Our studies reveal that miR-29a maintains HSC self-renewal and that Dnmt3a is a direct target of miR-29a that mediates the miR-29a-deficient hematopoietic phenotype. Thus, miR-29a is among a small but growing group of miRNAs shown to regulate HSC function. However, unlike other miRNA regulators of HSC function, which have been shown to regulate apoptosis or transforming growth factor-β signaling (miR-125),12,14 PI3K/AKT/GSK3β signaling (miR-126),15 or nuclear factor-κB signaling16 (miR-146), miR-29a appears to largely mediate its effects on HSC function by regulating the epigenetic state of HSCs by directly inhibiting expression of the DNA methyltransferase enzyme, DNMT3a.
The miR-29a/b-1-null phenotype is specifically due to loss of miR-29a because only ectopic expression of miR-29a, but not miR-29b, restored both the in vitro colony forming and long-term reconstitution ability of miR-29a/b-1-deficient HSPCs. These findings are consistent with our previous studies in which we demonstrated that overexpression of miR-29a, but not miR-29b, is sufficient to induce myeloid leukemia by conferring self-renewal to committed myeloid progenitors.9 Collectively, these findings provide further evidence that members of the same miRNA family may exert distinct biological functions. The biological basis of the unique effects of miR-29a and miR-29b in HSCs is not due to their tissue-specific expression, which has been shown for the thymus,19 but likely due to a unique 3′ hexanucleotide nuclear localization sequence in miR-29b, which has been shown to enhance its turnover during nonmitotic portions of the cell cycle.38
Dnmt3a is a primary mediator of the miR-29a-deficient hematopoietic phenotype, as shRNA-mediated reduction of Dnmt3a activity in miR-29a−/− HSPCs partially restored their in vitro colony formation capacity. In addition, deleting one allele of Dnmt3a in miR-29a/b-1+/− mice restored HSC function, demonstrated by recovery of HSC frequencies and their long-term reconstitution capacity. These studies are consistent with prior studies demonstrating the essential role of DNA methyltransferases in maintaining HSC function; loss of Dnmt3a resulted in expanded HSC numbers, impaired HSC reconstitution activity, and defects in differentiation,34,39 whereas ectopic Dnmt3a expression inhibited cell proliferation and survival.34 Additional data supporting the physiologic relevance of miR-29a regulation of Dnmt3a come from gene expression studies, which show that both miR-29a and Dnmt3a are highly enriched in HSCs.9,34 Thus, Dnmt3a is a physiologically relevant target of miR-29a, indicating that miR-29a regulates the balance between HSC self-renewal and differentiation/proliferation through a novel epigenetic mechanism. Nevertheless, the difference in HSC function between WT mice and miR-29a+/−;Dnmt3a+/− mice suggests that other miR-29a targets likely contribute to the miR-29a-null phenotype. Possible candidates contributing to this phenotype include predicted direct miR-29a target genes revealed by the miR-29a/b-1−/− HSC transcriptome including Cdk6,40 Tcl1,41 and Hbp1.9 Notably, miR-29a−/− HSCs exhibited dysregulated expression of numerous cell cycle regulators, reflecting their impaired ability to maintain quiescence, and it is likely that the observed increased cell cycling underlies, or at least reflects, the alteration in miR-29a−/− HSC function, as has been shown for other mouse mutants exhibiting increased HSC cycling and reduced function.42
We previously reported that miR-29a is expressed at high levels in human AML compared with purified HSPCs and that enforced expression of miR-29a in HSPCs is sufficient to drive a myeloproliferative phenotype and confer aberrant self-renewal to committed myeloid progenitors, supporting miR-29a as an important regulator of self-renewal.9 However, it appears that miR-29a’s function is cell context specific, as enforced expression of miR-29a or miR-29b suppresses AML cell proliferation and survival, providing a rationale for miR-29b-based therapies to treat AML patients.32,43 Thus, because miR-29a is critical for maintaining HSC self-renewal and miR-29 negatively influences AML cell growth, miR-29 agonist therapies would be expected to provide simultaneous negative growth effects on AML cells while promoting HSC self-renewal. However, given the presence of preleukemic HSCs in AML patients,44-46 whether or not such therapies may promote the growth or transformation of such preleukemic HSCs is of potential concern. Thus, examining the effect of miR-29-based therapies on preleukemic HSCs will be an important area of investigation in the future.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Agnes Viale of the Memorial Sloan Kettering Cancer Center Genomics Core; Dr Iannis Aifantis (New York University) for providing the Dnmt3a shRNA constructs; and Drs Ross Levine, Omar Abdel-Wahab, and Michael Kharas for helpful discussions and advice.
C.Y.P. is supported by National Institutes of Health, National Cancer Institute grant 1R01CA164120-01A1. B.d.S. is supported by a European Research Council advanced grant.
Authorship
Contribution: W.H. designed and performed research, analyzed data, and wrote the manuscript; J.D., S.M., B.d.S., and A.L. contributed the mouse model, designed the research, and wrote the manuscript; L.C. contributed the Dnmt3a knockdown constructs; D.C. and C.E.M. helped generate and analyze DNA methylation data; S.S.C. designed the research and wrote the manuscript; and C.Y.P. designed research, contributed reagents, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher Y. Park, 1275 York Ave, Box 20, New York, NY, 10065; e-mail: parkc@mskcc.org.

![Figure 7. Dnmt3a dysregulation mediates miR-29a/b-1-deficient HSC defects. (A) shRNAs (197 and 6567) efficiently knock down Dnmt3a mRNA levels by qRT-PCR in NIH 3T3 cells. (B) Knock down of Dnmt3a partially restores miR-29a/b-1-null HSPC function as assessed by methylcellulose colony formation assay. Bone marrow cells were harvested from fluorouracil (5-FU) treated mice and infected with either empty GFP+ retrovirus or shRNAs against Dnmt3a. GFP+ cells were double-sorted after 2 rounds of infection and plated in 6-well plates with 200 cells per well, and colony numbers were counted at day 12. (C) The 3- to 4-week-old progeny from miR-29a+/− Dnmt3awt/fl[Mx1-Cre] mice were treated with poly I:C 3 times every other day and were analyzed 1 month following the last injection. LSK (left) cells were significantly increased in miR-29a+/−;Dnmt3+/− mice, but committed progenitors were not affected (right). (D) Evaluation of cell cycle status of LSK cells in miR-29a+/−, Dnmt3a+/−, and miR-29a+/−;Dnmt3a+/− mice using a DAPI DNA stain. Shown with representative flow cytometry analysis (left) and summarized results (right). (E) Five million total bone marrow cells from miR-29a+/+;Dnmt3a+/+, miR-29a+/−;Dnmt3a+/+, miR-29a+/+;Dnmt3a+/−, and miR-29a+/−;Dnmt3a+/− mice were transplanted into lethally irradiated congenic recipients with 5 million competitor bone marrow cells. Peripheral blood donor chimerism was determined 4 months after transplant. (F) Bone marrow HSPCs were analyzed for donor cell chimerism. Error bars represent SEM from 5 to 12 mice for all experiments shown. Statistical significance was calculated using a Student t test: *P < .05; **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/14/10.1182_blood-2014-06-585273/4/m_2206f7.jpeg?Expires=1769227958&Signature=2SLMITJ3I0NiU2Vwu6a6CICh2flMGzvZxQJgYAOPvhPC2vxQZyrv4CGm8NnL7A7hzkxPIw8XkgeyVPUkxj2HIGvJ3QrDJDBLb9CDK6RIumIaHwlVVkcYB-1-M3xcBDRFUQ1CNr4yHehqCXWOmfasdrz5Z7yYQfgl4KftqsCQvU5WGGcnB3LDk1VVmv5ecODyMTrA8m5c4c0s1-c~cEVXEwloeV1VGaSyOxnsqT-xCwI8oZREZP3iy2LQArL1EpUTQUtLuteKatyGYsOTumncSApe3BwctLUhP4gIUb2ZyZRzPND-aK2gEc8QZFyOg3r8Me9vjsRI0J3QFYh9pZy~tQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)