Case presentation
M.T. is a 37-year-old whitemale with no significant past medical history who presented with sudden onset of tachypnea and chest pain, and was found to have hypoxia with oxygen saturation of 85% on room air. His heart rate was elevated to between 120 and 130 bpm, and his systolic blood pressure ranged from ∼100 to 110 mm Hg.On examination, he appeared to be uncomfortable due to dyspnea. A computed tomography angiogram of the chest showed extensive acute pulmonary emboli in bilateral main pulmonary arteries and a saddle embolism at the bifurcation. Echocardiogram revealed moderately enlarged right ventricle with reduced systolic function and right ventricular pressure elevated to 50 mm Hg, estimated from the gradient over the tricuspid valve. His troponin level was negative. He was immediately started on anticoagulation. He denied a recent history of surgery, long trip, immobilization, and major trauma. You were asked to evaluate this patient and wondered whether he would benefit from thrombolysis.
Introduction
Pulmonary embolism (PE) has an incidence rate of 60 to100 cases per 100 000 patients per year, with a 30-day case fatality rate of 10% to 30%.1-3 It accounts for at least 200 000 hospital discharges and 30 000 deaths each year.4 The standard of care for PE has been anticoagulation; the addition of thrombolysis may be beneficial but its effects remain controversial. In earlier studies, thrombolytic treatment demonstrated superior efficacy in clot resolution and improvement in hemodynamics compared with anticoagulation alone, leading to the approval of streptokinase, urokinase, and alteplase by the US Food and Drug Administration in the treatment of PE with hemodynamic instability (massive/unstable PE). However, the impact of thrombolysis on mortality has not been demonstrated definitively, given the relatively small number of patients enrolled in each randomized controlled trial (RCT). The increased risk of bleeding, on the other hand, has been shown repeatedly, so the net clinical benefit of thrombolysis in PE is debatable, particularly in patients who are hemodynamically stable (stable PE). A number of meta-analyses were recently published but reached different conclusions.5-8 The aim of this review is to provide evidence-based practice recommendations for the use of thrombolytic therapies in the treatment of PE with and without hemodynamic instability. In addition, we intend to determine the optimal regimen of thrombolytic therapy based on available data.
We made our recommendations using the guidelines suggested by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group (http://www.gradeworkinggroup.org) (see supplemental Appendix 1 on the Blood Web site).
Methods
We conducted the literature search in MEDLINE, EMBASE, the Cochrane Register of Controlled Trials, Web of Knowledge, and CINAHL databases, until July 20, 2014. We included RCTs comparing thrombolysis to anticoagulation alone and those comparing different types of thrombolytic agents in patients who had objectively confirmed symptomatic PE. We applied no limitations to language, publication date, patient age, or gender. We searched major international hematology and cardiology conference proceedings and abstracts in the past 10 years. Supplemental Appendix 2 lists the detailed search strategies. After selecting a final list of studies for data extraction, 3 authors (T.-F.W., A.S., and F.D.) independently reviewed each study and extracted data according to a predefined protocol. Discordances were resolved by consensus.
Our primary efficacy outcome was all-cause mortality during the follow-up period. Our primary safety outcome was the rate of major hemorrhage. Secondary outcomes included rate of recurrent PE and intracranial hemorrhage. Major hemorrhage events were reviewed and International Society on Thrombosis and Haemostasis (ISTH) criteria for major bleeding were applied when sufficient information was available; if not, major bleeding events were defined according to the original study. We performed the meta-analysis using the Review Manager v5.2 (Nordic Cochrane Center) according to the recommendations from the Cochrane Collaboration. We calculated odds ratios (ORs) and associated 95% confidence intervals (CIs) by the Mantel-Haenszel methods using the fixed-effect model. We tested the heterogeneity across studies by using I2. In the case of severe heterogeneity (I2 > 50%), we planned to use a random-effect model. We considered a 2-sided P value less than .05 to be statistically significant. The number needed to treat (NNT) and the number needed to harm (NNH) were calculated by dividing 1 by the absolute risk reduction. We assessed the risk of biases using the domains proposed by the Cochrane Handbook of Systematic Reviews of Interventions.9 Two reviewers (T.-F.W. and A.S.) independently scored the risk of biases; discordances were resolved by consensus. Funnel plots were used to assess publication biases (supplemental Appendix 3).
Results
Study selection progress is summarized in supplemental Appendix 4. A total of 33 studies were reviewed. There were 16 studies that compared thrombolysis to anticoagulation, and included a total of 2087 patients (Table 1).10-25 An additional 16 studies compared different types of thrombolytic agents, and included a total of 1244 patients (supplemental Appendix 5).26-41 One study (Ultrasound-Accelerated Thrombolysis of Pulmonary Embolism [ULTIMA] trial) investigated catheter-directed thrombolysis (CDT), and enrolled a total of 59 patients.42 All studies were randomized, but only 7 were clearly blinded.10,15,16,20,21,24,25 Allocation concealment and sequence generation were unclear in 3 studies.13,14,17 Here, we summarize our results in correspondence with several key questions we identified.
What are the benefits and risks of thrombolysis in patients with PE?
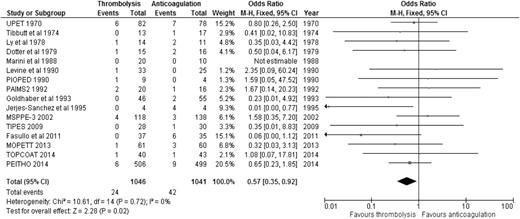
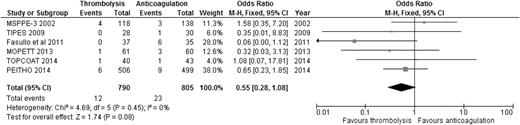
When the results of all RCTs were combined, regardless of the type of PE (stable or unstable), thrombolysis significantly reduced overall mortality compared to anticoagulation alone (2.29% [24/1046] vs 4.03% [42/1041]; OR: 0.57, 95% CI: 0.35-0.92) (Figure 1), with an NNT of 57. When we analyzed the 6 studies that included only patients with stable PE and clearly defined right ventricular dysfunction (RVD),20-25 the mortality benefit lost statistical significance, although a trend favoring thrombolysis over anticoagulation persisted (1.52% [12/790] vs 2.86% [23/805]; OR: 0.55, 95% CI: 0.28-1.08) (Figure 2), with an NNT of 75. In addition, when all studies were combined, thrombolysis significantly decreased the risk of PE recurrence (1.91% [19/995] vs 4.43% [44/993]; OR: 0.42, 95% CI: 0.24-0.72).
OR of overall mortality comparing thrombolysis to anticoagulation in stable PE with clearly defined RVD.
OR of overall mortality comparing thrombolysis to anticoagulation in stable PE with clearly defined RVD.
Jerjes-Sanchez et al19 conducted the only RCT to date that enrolled only patients who had massive PE and cardiogenic shock. Four patients were enrolled in each arm; however, the study was terminated after a marked difference in mortality was seen (0% in the thrombolysis group vs 100% in the heparin group, P = .02). The current standard of care of thrombolysis in unstable PE is thus determined, and no confirmatory studies are expected to be planned in this population, given ethical considerations.
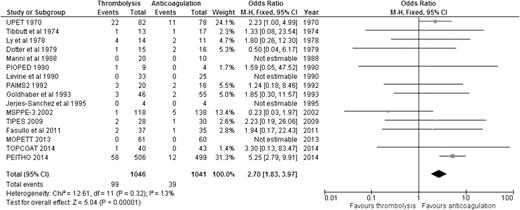
Regarding the risk of bleeding, our analysis showed that compared to anticoagulation alone, thrombolysis was associated with significantly increased risk of major bleeding (9.46% [99/1046] vs 3.75% [39/1041]; OR: 2.70, 95% CI: 1.83-3.97) (Figure 3), with an NNH of 18. Similarly, thrombolysis significantly increased the risk of intracranial bleeding vs anticoagulation (1.47% [15/1019] vs 0.20% [2/1013]; OR: 4.03, 95% CI: 1.41-11.53) (Figure 4), with an NNH of 78. When we considered the 6 studies enrolling only patients with stable PE and RVD, thrombolysis was associated with increased risk of major bleeding (OR: 3.56, 95% CI: 2.12-5.97) and intracranial bleeding (OR: 6.79, 95% CI: 1.51-30.50) when compared to anticoagulation alone (supplemental Appendix 6). It is worth noting that all RCTs excluded patients with high risk of bleeding, so these results should not be applied to those patients.
OR of major bleeding events comparing thrombolysis to anticoagulation.
OR of intracranial bleeding events comparing thrombolysis to anticoagulation. Analysis excludes 2 studies that did not report intracranial bleeding events.11,12
In conclusion, considering the risks and benefits of thrombolysis based on the available data, we recommend the following:
For patients with unstable PE, we recommend systemic thrombolysis (GRADE 1B).
For patients with stable PE and RVD, we suggest against routine use of systemic thrombolysis (GRADE 2B), given the lack of clear mortality benefit and increased bleeding risk. However, the use of thrombolysis could be considered in carefully selected patients at low risk of bleeding, particularly when the patient is persistently symptomatic.
For patients with stable PE and no RVD, we recommend against the use of systemic thrombolysis (GRADE 1B).
As stated above, our recommendations do not apply to patients with high risk of bleeding, given the lack of data in these patients. In the absence of high-quality data, major international organizations such as the European Society of Cardiology have published practice guidelines to address these difficult scenarios.43 Common absolute contraindications to systemic thrombolysis by consensus include hemorrhagic stroke, ischemia stroke within 6 months, central nervous system damage or neoplasms, trauma or surgery within 3 weeks, gastrointestinal bleeding within a month, and known bleeding disorders.
What is the best thrombolytic agent?
Alteplase, tenecteplase, urokinase, and streptokinase are the main thrombolytic agents investigated in RCTs. Sixteen RCTs directly compared different types and dosing regimens of thrombolysis (supplemental Appendix 5), but no definitive conclusions could be made, given the large variety of regimens used.
In conclusion, there is no evidence to suggest that one thrombolytic agent is superior over others.
Do different doses of thrombolytic agents matter?
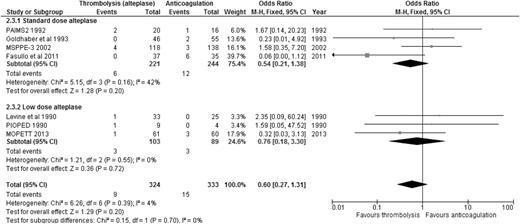
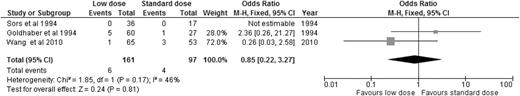
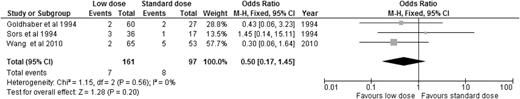
Alteplase is the best-studied thrombolytic agent in different dosing regimens. Therefore, we performed an analysis of all the RCTs using alteplase as the thrombolytic agent, aiming to answer this question. When we compared alteplase, subdivided by standard vs low dose, to anticoagulation alone, there was no significant difference in overall mortality or major bleeding for either dosing regimen (Figures 5 and 6). We then analyzed the 3 studies directly comparing low-dose (up to 50 mg) to standard-dose (100 mg) alteplase.32,33,40 There was no statistically significant difference in overall mortality or major bleeding, although low-dose alteplase showed a trend toward reduced major bleeding events (OR: 0.50, 95% CI: 0.17-1.45) (Figures 7 and 8). These results were slightly different from a similar meta-analysis done by Zhang et al,44 who found a significant reduction in major hemorrhage with low-dose alteplase, with no difference in all-cause mortality. This difference was attributed to a slight difference in the number of major bleeding events included in their meta-analysis. We elected to use the number reported by the original manuscript because we believed there were insufficient data to allow application of ISTH criteria and reassignment of major bleeding events. Due to the small sample size, these conclusions are hypothesis generating, and require further confirmation in large clinical trials.
ORs of overall mortality comparing alteplase to anticoagulation, subcategorized by standard-dose vs low-dose alteplase.
ORs of overall mortality comparing alteplase to anticoagulation, subcategorized by standard-dose vs low-dose alteplase.
ORs of major bleeding events comparing alteplase to anticoagulation, subcategorized by standard-dose vs low-dose alteplase.
ORs of major bleeding events comparing alteplase to anticoagulation, subcategorized by standard-dose vs low-dose alteplase.
OR of overall mortality in studies directly comparing low-dose to standard-dose alteplase.
OR of overall mortality in studies directly comparing low-dose to standard-dose alteplase.
OR of major bleeding events in studies directly comparing low-dose to standard-dose alteplase.
OR of major bleeding events in studies directly comparing low-dose to standard-dose alteplase.
In conclusion, low-dose alteplase showed similar efficacy and safety compared to standard-dose alteplase. However, currently available evidence is insufficient to recommend low-dose alteplase as the standard of care. Therefore, when alteplase is used, we recommend using standard-dose over low-dose (GRADE 2B). However, low-dose alteplase can be considered for patients at high risk of bleeding when no alternative treatment strategies are available.
What about CDT?
Systemic thrombolysis is associated with significant risk of bleeding, and thrombolytic modalities with minimal bleeding risk are desirable. Among them, CDT appears promising. CDT is expected to deliver thrombolytic agents in high concentration to the site of thrombosis, allowing maximal thrombolytic effects while minimizing systemic exposure to thrombolysis and subsequent bleeding. Up to this date, the ULTIMA trial42 is the only RCT comparing CDT to anticoagulation. This trial enrolled 59 patients with stable PE and RVD in an open-labeled design. The primary objective was to determine whether interclot delivery of ultrasound-assisted CDT could improve right heart function at 24 hours compared to anticoagulation alone. The investigators found that ultrasound-assisted CDT significantly reversed right ventricular dilatation at 24 hours, with no major hemorrhage. The sample size was too small to evaluate for mortality benefit. Another meta-analysis of 35 cohort studies (594 patients) employing CDT for the treatment of massive PE demonstrated a clinical success rate of 86.5% and a major procedural complication rate of 2.4%.45 These investigators concluded that CDT was safe and effective in treating massive PE; however, no RCTs were included in the meta-analysis, indicating the need for such studies.
In conclusion, given the lack of sufficient evidence, we suggest that when thrombolysis is considered for PE, CDT should not be considered as the standard of care over systemic thrombolysis at this time (GRADE 2C).
Discussion
The use of thrombolytic agents for PE patients, especially those with stable hemodynamics, remains controversial despite multiple RCTs. In 2014, at least 4 meta-analyses were published, all aiming to investigate the efficacy and safety of thrombolysis.5-8 Surprisingly, they reached slightly different conclusions. Both Cao et al8 and Nakamura et al5 analyzed only studies of stable PE and concluded that thrombolysis failed to improve overall mortality or recurrent PE with similar risk of major bleeding. However, Marti et al6 and Chatterjee et al7 found a significant reduction in overall mortality with thrombolysis when all PE studies were combined, with increased risks of major hemorrhage and intracranial bleeding. When the subset studies of stable PE were analyzed, the significant reduction in mortality disappeared in the analysis by Marti et al6 but persisted in that of Chatterjee et al. 7 The different results of these meta-analyses were due to the differences in study inclusion and statistical methodologies. Table 2 summarizes the key differences among these 4 meta-analyses and compares them to our own analysis. In general, the meta-analyses including only studies of stable PE reached different conclusions from those including all studies regardless of the type of PE.
From the efficacy perspective, thrombolysis was shown to reduce overall mortality in the meta-analyses that included all PE. When only stable PE was considered, no mortality benefit was shown, except in the analysis by Chatterjee et al.7 This study was the only one that used the Peto method for analysis. The Peto method works well when intervention effects are small (ORs are close to 1) but could give biased results in other situations.46 We therefore chose to use the Mantel-Haenszel method for our analysis, as what was done in other meta-analyses. This difference in statistical methodology could have contributed to our different conclusions.
From the safety perspective, thrombolysis was shown to increase the risk of major bleeding in all 3 meta-analyses including all PE. Thrombolysis also increased the risk of major bleeding in stable PE in the analysis done by Chatterjee et al7 and in ours, but not in the analyses done by Cao et al8 and Nakamura et al.5 Cao et al8 did not include the PEITHO study (the largest study),25 because the meta-analysis was done prior to the publication of the PEITHO study. Fewer patients were therefore included, which could have resulted in loss of power. Nakamura et al5 used the Mantel-Haenszel random-effect models, which are known to be more conservative in the calculation46 and could have contributed to the different conclusions reached. We chose to use fixed-effect models because there was low heterogeneity across the included studies. Furthermore, in our analysis of stable PE with RVD, the studies we included were not identical to the ones in the analyses done by Cao et al8 and Nakamura et al5 (Table 2). We chose to include only studies in which RVD was clearly defined, in order to draw conclusions in this particular patient population.
Several points are worth noting in our analysis. First, studies included in our meta-analysis had a large variation in the follow-up duration (3-840 days). To adjust for this variable, we performed a subgroup analysis separating studies into groups of different follow-up duration (≤30 and >30 days). We found that follow-up duration did not affect the main outcomes (overall mortality or major bleeding), likely due to the fact that all but 2 studies22,23 had relatively short follow-up periods (≤30 days). The seemingly large variation of follow-up periods, therefore, did not play a major role.
Second, the definitions of major bleeding varied in each included study. We tried to adopt the ISTH criteria for major bleeding whenever possible in our analysis, but missing details from original manuscripts precluded application of the criteria in many cases. In these scenarios, the numbers of events reported in the original manuscript were used. We acknowledge that this may account for some of the differences in our results compared to those of other meta-analyses. Lastly, funnel plots revealed a potential risk of publication bias in reporting major bleeding events but not in the analysis of overall mortality (supplemental Appendix 3).
In summary, this study aims to provide clinical guidance on the use of thrombolysis in PE. We have based our recommendations on the evidence derived from the analysis of a comprehensive list of RCTs. In addition to the critical analysis to address the most commonly encountered dilemma; that is, the need for thrombolysis, we attempt to answer other important clinical questions in a systematic, evidenced-based manner, including the optimal type and dose of thrombolytic agents and the role of CDT. We found that (1) thrombolysis reduced overall mortality in all PE but not in stable PE with clearly defined RVD; (2) thrombolysis consistently increased major bleeding and intracranial bleeding events; (3) no single thrombolytic agent has shown superiority over another; (4) although low-dose alteplase may potentially reduce bleeding risk, the data are insufficient to suggest its routine use; and (5) CDT is promising, but more studies are needed before it can be recommended routinely.
The patient presented here did not receive systemic thrombolysis after interdisciplinary discussions between medicine, pulmonary, hematology, and interventional radiology teams because he remained hemodynamically stable throughout the hospitalization. His respiratory symptoms improved with anticoagulation only, and he did not require oxygen on discharge. He was discharged home with warfarin therapy. Six months after the event, he was doing well without recurrent PE.
The online version of this article contains a data supplement.
Authorship
Contribution: T.-F.W. conducted the literature search, data extraction and analysis, and quality assessment, and wrote the first draft of the manuscript; A.S. performed the literature search, data extraction and analysis, and quality assessment; F.D. performed the data extraction; W.A. conceived the study; and all authors provided input to the study design and critical review and revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Walter Ageno, Short Medical Stay Unit and Thrombosis Center, Ospedale di Circolo e Fondazione Macchi, viale Borri 57, 21100 Varese, Italy; e-mail: agewal@yahoo.com.