Key Points
The first wide, prospective report on the role of IEM in the differential diagnosis of systemic amyloidosis.
IEM allows for the correct characterization of the amyloid protein in virtually all cases and represents a viable alternative to mass spectrometry.
Abstract
Accurate diagnosis of systemic amyloidosis is necessary both for assessing the prognosis and for delineating the appropriate treatment. It is based on histologic evidence of amyloid deposits and characterization of the amyloidogenic protein. We prospectively evaluated the diagnostic performance of immunoelectron microscopy (IEM) of abdominal fat aspirates from 745 consecutive patients with suspected systemic amyloidoses. All cases were extensively investigated with clinical and laboratory data, with a follow-up of at least 18 months. The 423 (56.8%) cases with confirmed systemic forms were used to estimate the diagnostic performance of IEM. Compared with Congo-red–based light microscopy, IEM was equally sensitive (75% to 80%) but significantly more specific (100% vs 80%; P < .001). In amyloid light-chain (AL) amyloidosis, κ cases were more difficult to diagnose (sensitivity 71%), whereas the analysis of abdominal aspirate was informative in only 40% of patients with transthyretin amyloidosis. We found a high prevalence (20%) of a monoclonal component in patients with non-AL amyloidosis, highlighting the risk of misdiagnosis and the need for unequivocal amyloid typing. Notably, IEM identified correctly the specific form of amyloidosis in >99% of the cases. IEM of abdominal fat aspirates is an effective tool in the routine diagnosis of systemic amyloidoses.
Introduction
Amyloidosis is a heterogeneous group of diseases that share the deposition of amyloid fibrils in organs and tissues, with the same characteristic cross–β-sheet secondary structure, independently of their protein primary structure.1 More than 30 unrelated autologous proteins can produce systemic amyloidoses,2-5 either localized or systemic. The various forms differ in pathogenesis and prognosis, but they usually show overlapping clinical manifestations, making their differentiation on clinical grounds very difficult. Precise amyloid typing is crucial for the adequate treatment of patients because the various forms require different approaches, which can range from autologous stem cell transplantation in amyloid light-chain (AL) amyloidosis to liver transplantation in transthyretin (TTR) amyloidosis (ATTR).2,4,5
Diagnosis and classification are based on histologic demonstration of amyloid deposits and characterization of the amyloid precursor. Abdominal subcutaneous fat aspiration with a fine needle is fast and harmless and is the most common diagnostic tool when a systemic form is suspected,6 offering a convenient alternative to organ biopsy. The resulting tissue smear is examined by polarized light microscopy (LM) after Congo red staining in order to detect the presence of amyloid. The second step is to identify the amyloidogenic protein in order to unequivocally establish the type of amyloidosis. Traditional histochemistry-based typing techniques have shown relevant limitations,7-10 with improved results in organ biopsy specimens examined in a highly specialized laboratory in a recent series.11 Mass spectrometry techniques8 have paved the way for novel automated proteomic assays that are now considered a diagnostic standard,12-14 but this complex and expensive technology is not yet available in most institutions.
Immunoelectron microscopy (IEM) is a technique that combines immunohistochemistry and electron microscopy. Using gold-labeled secondary antibodies, IEM can colocalize the protein within amyloid fibrils and greatly reduce background staining, which is the most common cause of reduced specificity in immunohistochemistry.15 As a reference center in Italy, we developed a program devoted to the immune characterization of amyloid in abdominal fat, based on the routine use of electron microscopy and IEM.16,17 In a small series, we previously reported that IEM of abdominal fat can reliably characterize amyloid deposits in suspected cardiac amyloidosis.18 The aim of the present study was to prospectively evaluate the diagnostic value of IEM in a consecutive series of abdominal fat aspirates from patients with suspected systemic amyloidoses.
Methods
Patients
This was a prospective study enrolling 745 consecutive patients (432 men and 313 women; median age, 63 years; range, 24 to 89 years) referred to the Amyloidosis Research and Treatment Center, Foundation IRCCS Policlinico S. Matteo, Pavia, for suspected systemic amyloidosis from May 2003 to December 2010. Patients were referred for suspected cardiac (332), renal (360), peripheral or autonomous nerve (130), liver (50), soft tissue (152), gastrointestinal (26), and/or other organ involvement (56) in systemic amyloidosis. Two hundred and seventy-two (36.5%) patients had involvement of 2 or more suspected organs. Baseline demographics and clinical and laboratory data, including possible organ involvement, were recorded. Abdominal fat aspiration was performed in all patients, and samples were submitted for LM and IEM. Biopsy specimens from other organs besides abdominal fat tissue were available for IEM in 323 cases. Analysis of the genes encoding TTR, apolipoproteins A1 and A2, fibrinogen, and lysozyme was performed by automated sequencing of all gene-coding regions.19,20 All patients were followed for at least 18 months; 22 (2.9%) were lost to follow-up and therefore excluded from the study. At the end of follow-up, all patient data were reviewed by the study clinicians (G.M. and G.P.), and the diagnosis and type of amyloidosis were confirmed or rejected on the basis of clinical and laboratory findings. Written informed consent was obtained for all patients, in accordance with the Declaration of Helsinki, for the use of biological samples and clinical data for research purposes, according to institutional review board guidelines.
LM examination
IEM
Specimens were fixed by immersion in a modified Karnovsky solution (0.5% glutaraldehyde, 2% paraformaldehyde in 0.2 M cacodylate buffer [pH 7.3]) for at least 4 hours and postfixed in 1% osmium tetroxide in the same buffer. Samples were then dehydrated through a graded series of ethyl alcohols and embedded in epoxy resin. Ultrathin (600- to 800-Å-thick) sections were cut, mounted on nickel grids, and stained with 5% uranyl acetate and lead citrate (Reynolds solution). A minimum of 5 sections for each patient were observed with a Philips CM12 electron microscope. Selected sections were then processed for postembedding immunogold as previously reported.16 Briefly, sections were etched with 3% H2O2 for 10 minutes at room temperature. Anti-κ light chain and anti-TTR immunostain required enzymatic predigestion (0.05% trypsin in Tris buffer with 0.05% CaCl2, 37°C, 15 minutes) to unmask antigenic epitopes. The section were then rinsed in 0.05 M Tris/HCl buffer (pH 7.3), incubated with either 1:20 normal goat serum or 1% egg albumin for 15 minutes at room temperature and subsequently overnight at 4°C with the primary antibodies (Dako, Glostrup, DE), rinsed with the same buffer, and then incubated for 60 minutes at room temperature with the secondary antibody (anti-rabbit or anti-mouse immunoglobulin G) or with protein-A, both of which were conjugated to 15-nm colloidal gold particles (British Biocell International, Cardiff, UK). The panel of primary antibodies covers the commonest forms of amyloidosis: AL (κ and λ), amyloid A (AA), and ATTR; in selected cases, immunogold labeling was performed also with antibodies directed against apolipoprotein A1. Fibrinogen and lysozyme were investigated when clinically indicated. Sections were stained with 5% uranyl acetate and lead citrate (Reynolds solution) and scanned with a Phillips CM12 electron microscope (Figure 1). The specificity of immunoreactions was verified using either normal goat serum or egg albumin instead of primary antibody.17,18 Each reaction was also checked by immunostaining known positive samples with primary antibodies.
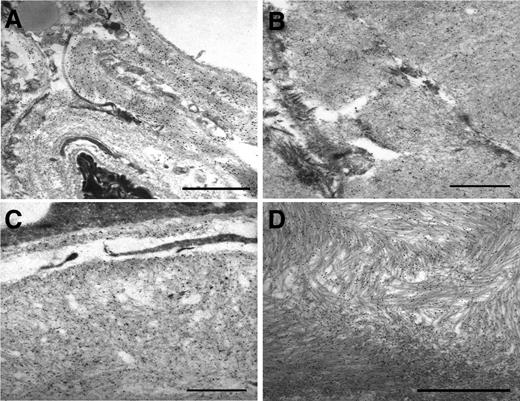
IEM of abdominal fat aspirates from patients with different amyloid diseases. Postembedding immunostaining with polyclonal anti-λ light chains (A), anti-k light chains (B), and monoclonal anti-SAA (C) and polyclonal anti-TTR (D) antibodies. Secondary antibodies are conjugated with gold particles 15 nm in diameter. Uranyl acetate, lead citrate. Scale bar, 1 μm.
IEM of abdominal fat aspirates from patients with different amyloid diseases. Postembedding immunostaining with polyclonal anti-λ light chains (A), anti-k light chains (B), and monoclonal anti-SAA (C) and polyclonal anti-TTR (D) antibodies. Secondary antibodies are conjugated with gold particles 15 nm in diameter. Uranyl acetate, lead citrate. Scale bar, 1 μm.
Data analysis
Descriptive statistical analysis of patient demographic, clinical, and laboratory data was performed. Sensitivity, specificity, and predictive values of LM and electron microscopy were assessed against the gold standard of expert clinical evaluation on patient charts and follow-up. The diagnostic performance of the tests was compared with respect to amyloid types and involved organs with the Pearson χ2 test. Agreement analyses between IEM and final diagnosis were performed using κ statistics, and exact logistic regression was used to identify predictors of disagreement. Statistical tests were performed with PASW software 18.0 for Windows (Chicago, IL) and STATA 13.1 (Stata Corp, College Station, TX). P < .05 was required for statistical significance.
Results
Overall diagnostic performance of LM and electron microscopy
After extensive clinical and laboratory analysis, 458 out of 745 patients (61.5%) were diagnosed with amyloidosis. In the remaining 287 patients, the absence of any evidence of amyloidosis and a clinical follow-up of at least 18 months allowed us to rule out the diagnosis. Taking into account only systemic forms, 423 (56.8%) positive cases were included in the study. The distribution and characteristics of the different forms of amyloidosis are summarized in Table 1. The diagnostic performance, in terms of sensitivity, specificity, and predictive values, is reported in Table 2. Both techniques showed good sensitivity (79% and 76.1%, respectively), but IEM was significantly more specific than LM (100% vs 79.7%, P < .001). Forty-five patients with systemic amyloidosis and positive abdominal fat results with LM were negative with IEM: 33 with AL amyloidosis (23 λ, 10 κ), 7 with ATTR, 4 with AA amyloidosis, and 1 with fibrinogen amyloidosis. The diagnosis in these cases was based on the characterization of amyloid deposits in 31 biopsy specimens from other organs besides fat tissue (20 renal, 3 liver, 3 salivary glands, and 2 bone marrow, 1 lymph node, 1 endomyocardial, and 1 rectal) and/or DNA sequencing (8 cases). Of the 89 patients with negative LM findings in whom a diagnosis of amyloidosis was finally achieved, 33 had a positive fat aspirate result with IEM. These comprised 28 AL (21 λ, 7 κ) and 5 AA cases. In the other 56 cases, systemic amyloidosis was diagnosed by biopsy of 1 or more affected organs (33 renal, 9 endomyocardial, 4 liver, 4 nerve, 4 gastrointestinal, 3 lymph nodes, 2 bone marrow, 1 salivary gland and 1 pulmonary) and/or DNA sequencing (12 cases).
Performance of IEM in amyloid typing
IEM correctly identified the specific form of amyloidosis in >99% of the cases. The agreement between IEM results and final diagnosis was substantial (κ = 0.79; P < .001). Gender was a significant cause of disagreement, with males having a higher probability of false-negative results (P < .001, at logistic regression); age at diagnosis was not significant in this regard.
Only in 2 out of 423 positive cases (0.5%) was the amyloid component misclassified by IEM. One case involved a 73-year-old male patient with no history of chronic inflammation, suspected heart and kidney involvement (including proteinuria and renal failure), and κ light-chain M-protein by immunofixation. IEM showed κ-immunoreactive deposits associated with weak positivity for serum AA; proteomic analysis of abdominal fat identified AA amyloidosis. No specific inflammatory disease could be diagnosed. The second case involved a 76-year-old man with isolated cardiac involvement and κ light-chain M-protein by immunofixation. IEM was positive for κ light chains; however, the Ile68Leu DNA mutation in the TTR gene was found. ATTR amyloidosis was finally confirmed by proteomic analysis of abdominal fat.
The presence of monoclonal components in serum or urine was a potential confounding factor in the diagnosis of patients with non-AL systemic amyloidoses. Of 103 patients with non-AL systemic amyloidoses, 22 had a monoclonal component in serum and/or urine (21.4%). The final diagnosis was AA (16) and mutated ATTR (6) amyloidosis. The predominantly involved organs in these patients were the heart (13), kidney (15), and peripheral/autonomous nervous system (9).
The presence of macroglossia was a positive predictor for AL amyloidosis diagnosis. Thus, among 39 patients with macroglossia and positive LM findings, 38 (27 κ/11 λ) were diagnosed with AL amyloidosis. Only 1 patient with chronic arthritides and AA amyloidosis had macroglossia.
Diagnostic performance by amyloid type
Systemic AL amyloidosis
Three hundred twenty patients (76% of systemic amyloidoses) were diagnosed with AL amyloidosis, mainly of λ isotype (68.9%). Serum and/or urine immunofixation results were positive in 96.3% of the patients with AL amyloidosis, and a serum or urine monoclonal component was more frequently found by immunofixation in patients with λ (99.1%) than in those with κ AL amyloidosis (89.9%; P < .001). The addition of the serum free light-chain (FLC) ratio together with serum and urine immunofixation allowed the identification of a monoclonal protein in 99.4% of AL patients.
As reported in Table 3, LM and IEM of abdominal fat aspirates diagnosed ∼80% of the patients with AL amyloidoses, but the sensitivity in AL κ cases (74% and 71%) was significantly lower in comparison with λ (84% and 83%) for both techniques (P = .03 and P = .016, respectively). The diagnostic performance of the 2 techniques also differed according to the involved organ. Abdominal fat LM and IEM were more sensitive in patients with cardiac involvement than in patients with noncardiac amyloidosis (89.7% vs 77.3%; P < .001) and less sensitive in patients with any renal involvement than in other patients (74.4% vs 83.3%; P = .037).
Predictors of disagreement at logistic regression were AL type and specific organ involvement, together with gender; AL κ type was shown to be associated with a higher rate of disagreement in IEM than AL λ (P < .001, sex-adjusted model). Also, the rate of disagreement was lower in the presence of isolated heart involvement (P = .047, sex-adjusted model) and higher in the presence of kidney involvement (P = .019, sex-adjusted model).
AA amyloidosis
AA amyloidosis was the second most common type in this series. Reactive amyloidosis occurred in rheumatoid arthritis (13 patients), inflammatory bowel disease (8 patients), other polyarthritis types (14 patients with ankylosing spondylitis, polymyalgia rheumatica, psoriatic arthritis, and others), familial periodic fever syndrome (4 patients), Castleman disease (2 patients), polyserositis (2 patients), Schnitzler syndrome (2 patients), cystic fibrosis (2 patients), and other inflammatory conditions (5 patients). In 17 out of 69 patients (25%), it was not possible to identify any underlying cause. LM and IEM sensitivities (77%) were very similar to those observed in AL amyloidosis (Table 3); the sensitivity of IEM for AA amyloidoses was significantly higher when compared with that for ATTR (77% vs 43%; P = .002). A monoclonal protein was found by immunofixation in almost one-quarter of the AA patients. Five patients with a negative fat aspirate finding by LM and IEM were confirmed to have AA amyloidosis by IEM on different organ biopsy specimens (3 kidney, 1 endomyocardium, and 1 minor salivary gland).
ATTR amyloidosis and other familial forms
Most ATTR amyloidosis cases in the present series were due to mutations in the TTR gene (25 patients), whereas only 5 senile wild-type ATTR forms were identified. The reason for this relatively low number of ATTR amyloidosis cases in our series is that in patients with a positive family history of ATTR amyloidosis and consistent clinical presentation, the identification of the mutation is often considered sufficient to make the diagnosis.23 The most frequent mutations were Val30Met (7 patients), Ile68Leu (4 patients), Tyr78Phe (3 patients), and Val122Ile (3 patients). Abdominal fat LM sensitivity for these forms was slightly lower (particularly in senile forms) than for AL and AA amyloidosis. IEM sensitivity was significantly lower for ATTR than for the other types. Thirty-three other patients were diagnosed with ATTR in our center during the study period by means of gene sequencing, but without IEM analysis, fat aspirate LM sensitivity for ATTR amyloidosis reached 71.4% (95% confidence interval [CI], 58.5-81.8). A monoclonal protein was found by immunofixation in 24% of patients with mutated ATTR. The diagnosis in 3 out of the 5 patients with senile ATTR forms was confirmed by IEM on endomyocardial biopsy specimens.
The other forms of familial amyloidosis included 2 apolipoprotein A1 mutations (Leu75Pro), 1 lysozyme mutation (Trp64Arg), and 1 case of renal involvement by fibrinogen amyloidosis (Glu526Val). Only this last case was positive by LM analysis of fat aspirates, and all cases were negative by IEM.
Proposed approach to the diagnosis of systemic amyloidoses
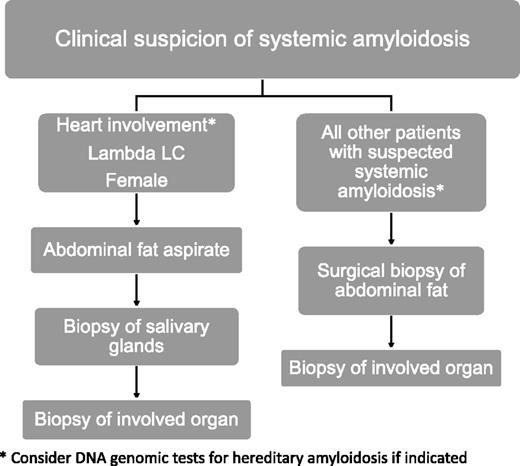
According to our models used to identify predictors of disagreement, the patients with a higher risk of a false-negative result are those with AL κ-type amyloidosis, renal involvement (particularly if isolated), and/or male gender. Accordingly, we recommend a practical approach to the diagnosis of systemic amyloidosis as reported in Figure 2, which takes into account other biopsy specimens in addition to fat aspirates, such as surgical abdominal fat samples, labial salivary glands, or biopsies of involved organs. For instance, we have previously shown that the biopsy of minor salivary glands has a 58% diagnostic sensitivity in patients with negative abdominal fat aspirate results, and the combined negative predictive value of the 2 biopsies is >90%.24 However, the biopsy of clinically affected organs can be required in selected cases to achieve the final characterization of amyloid deposits.
Suggested diagnostic approach and fat tissue biopsy procedure in patients with suspected systemic amyloidoses according to gender, organ involvement, and involved light-chain isotype. Genetic testing for hereditary amyloidosis is indicated in patients with isolated cardiac (to exclude TTR and apolipoprotein A1 amyloidoses) or renal (for fibrinogen and lysozyme) involvement, as well as in those with peripheral neuropathy, either isolated or associated with cardiac involvement (TTR). LC, light chain.
Suggested diagnostic approach and fat tissue biopsy procedure in patients with suspected systemic amyloidoses according to gender, organ involvement, and involved light-chain isotype. Genetic testing for hereditary amyloidosis is indicated in patients with isolated cardiac (to exclude TTR and apolipoprotein A1 amyloidoses) or renal (for fibrinogen and lysozyme) involvement, as well as in those with peripheral neuropathy, either isolated or associated with cardiac involvement (TTR). LC, light chain.
Discussion
This is the largest study on diagnostic performance of abdominal fat and any other biopsy site in systemic amyloidosis. Abdominal fat analysis by IEM allowed us to reach a definitive diagnosis in 79.4% of AL cases, 76.8% of AA cases, and 43.3% of ATTR amyloidosis cases. The sensitivity of IEM on abdominal fat aspirates in our tertiary reference center was 76.1%, with 100% specificity and 99% correct classification of the amyloid type. Sensitivity was, however, higher in AL λ amyloidosis, the most frequent protein type. In a recent report, 110 out of 117 cases (94%) were correctly classified using immunohistochemical stains of involved organ biopsy specimens, with 96% sensitivity and 100% specificity in a subgroup of patients with a known amyloid type.11 In our study, IEM of abdominal fat aspirates yielded similar specificity (100%) and a slightly lower sensitivity (80%), and it correctly typed the amyloid deposits in 99.5% of cases. In their study, all the biopsy specimens studied were “positive.” However, in the cited report,11 calculation of sensitivity and specificity was done only in a subgroup of 51 patients in whom they were confident enough about the final diagnosis. The 94% figure is the proportion of patients classified in the whole group, including cases lacking a clinical final diagnosis.
Using abdominal fat aspirate samples for amyloid typing has several relevant advantages over organ biopsy samples: fat aspiration is usually performed on the same day of the clinical consultation, and time needed for completing the IEM typing is ∼7 to 10 days, allowing for prompt therapy initiation, which is particularly important in AL amyloidosis. Abdominal fat is the elective biopsy site because it is easily accessible, minimally invasive, and can be repeated during follow-up, if necessary.25,26 In cases where more tissue is needed due to focal deposition of the amyloid fibrils, a surgical abdominal fat biopsy allowing a larger sample might be indicated. Although it has been reported that once a hematostatic disorder is appropriately ruled out, bleeding risk during kidney biopsy is not increased in patients with systemic amyloidosis,27 liver biopsy carries a significant risk of bleeding that may have fatal consequences. Liver biopsy should be avoided or undertaken via the transjugular route. Furthermore, some biopsies, such as endomycardial biopsies, can be performed only in specialized centers. Moreover, abdominal fat aspirate is also suitable for the analysis of amyloid protein composition through proteomics, which is becoming an interesting source of information on the interaction between amyloidogenic and nonamyloidogenic proteins in tissue.12,28 In this series, proteomics was useful in 2 cases, 1 AA and 1 ATTR, misdiagnosed as AL due to nonspecific precipitation of serum immunoglobulin on the amyloid deposits.14,29
As previously stated, the correct typing of amyloid deposits is essential for patient management. Several techniques have been developed for typing, such as enzyme-linked immunosorbent assay, western blot, immunohistochemistry, IEM, or mass spectrometry.30 Mass spectrometry is now considered a standard for amyloid typing, allowing the unequivocal identification of the amyloid protein, and is independent of availability of specific antibodies. It can be done profitably when centralized and even performed on paraffin-embedded fat samples. However, dedicated mass spectrometers are very expensive, require highly qualified personnel, and are available in very few institutions worldwide. Our IEM protocol utilizes commercial antibodies and can be applied in the referral hospital’s pathology laboratory. However, only certain amyloidosis units apply IEM systematically. Therefore, specificity and positive predictive value may not be as good in other centers with less experience with this technique. IEM allows the “in situ” correlation of antibody localization and fibril morphology, overcoming the limit of nonspecific stain of light immunohistochemistry and of custom-made antibodies used in other studies.11
The diagnostic performance in the general series was good; all patients with positive IEM results were affected by the systemic form of amyloidosis, with 100% positive predictive value. Diagnostic agreement was significantly lower in men. The negative predictive value was 74%. This relatively low negative predictive value could be the consequence of the sparse, focal distribution of less abundant amyloid deposits, which can be absent in small samples of fat aspirates of patients with systemic amyloidosis. For this reason, when systemic amyloidosis is strongly suspected on clinical grounds and the abdominal fat aspirate result is negative, the diagnostic hypothesis should not be ruled out. Thus, diagnostic performance of abdominal fat aspirates in male patients with AL κ-type amyloidosis and renal involvement is suboptimal, and in these patients, a surgical biopsy from abdominal subcutaneous fat may be more informative31 (Figure 2).
As far as amyloid typing is concerned, AL λ cases were the most easily detected by IEM, in contrast to κ cases, where IEM sensitivity was significantly lower. This previously unreported observation suggests that negative IEM results should be considered carefully when κ-type AL amyloidosis is suspected. In our experience with 2 recent cases of κ light-chain monoclonal components, κ light chains could be identified in abdominal fat aspirates by proteomics; IEM was negative in one of them, and the result was nonconclusive in the other.29 LM positivity correlated with the number of involved organs, a tendency not confirmed by IEM. Independently of light-chain isotype, patients in whom the kidney was the predominantly involved organ were more likely to have negative abdominal fat aspirate results than those affected by diffuse systemic or predominantly cardiac involvement. Of note, high sensitivity of IEM on fat tissue for cardiac amyloidosis has been described previously.18 These observations should be taken into account when choosing the best diagnostic approach and interpreting test results in any given patient (Figure 2).
Interestingly, IEM analysis of abdominal fat aspirates was less sensitive (43%) in patients with ATTR amyloidosis. This may be due to the focal pattern of amyloid deposition in ATTR amyloidosis,23,32 which raises the possibility of false-negative results in small samples of fat tissue. On the other hand, IEM has a less crucial role in the diagnosis of hereditary ATTR amyloidosis, because in patients with a known family history, the diagnosis could be supported by DNA analysis.23 In patients with senile systemic amyloidosis, cardiac biopsy remains the gold standard, because the abdominal fat aspiration has shown low sensitivity. It has been reported, in a previous small series of 11 patients, that a surgical skin biopsy including the deep subcutaneous fat pad can be useful for the histopathological diagnosis of senile systemic amyloidosis.33 Scintigraphy with 99mTc 3,3-diphosphono-1,2-propanodicarboxylic acid or pyrophosphate can also provide useful diagnostic hints in an appropriate clinical context.34,35
The prevalence of hereditary amyloidosis (ATTR) associated with a benign monoclonal gammopathy reached 24%. This phenomenon has been recognized with variable prevalence in other series, ranging from 3 out of 7 patients36 to “low-grade” monoclonal gammopathies in 24% of familial amyloidosis patients,10 as in our series. This high prevalence may be due to a referral bias, because most patients are referred to our hematology-oriented center. It is worth emphasizing that IEM was able to correctly classify >99% of these patients. Only 2 cases had a nonspecific reaction to κ antibody, possibly due to entrapment of serum immunoglobulins within the amyloid fibrils, whereas proteomic unequivocally classified them as ATTR and AA amyloidosis. On the other hand, 99% of AL λ and 90% of AL κ amyloidosis patients had a detectable monoclonal component by serum or urine immunofixation, which increased to 99% when serum FLC was combined with immunofixation. This means that AL κ cases are more frequently oligosecretory, as has already been reported in multiple myeloma.37 This subgroup of patients would be more difficult to diagnose by either immunofixation38 or IEM, with a more relevant diagnostic value of serum FLC ratio determination in these cases.
In conclusion, IEM increased to 100% the specificity of abdominal fat aspirate analysis for the diagnosis of systemic amyloidosis, with a high level of agreement. IEM can correctly characterize amyloid deposits in 99.5% of cases, thus representing an efficient and more readily available alternative to mass spectrometry. The high prevalence of a monoclonal component in patients with non-AL amyloidosis emphasizes the risk of misdiagnosis and the need for an unequivocal amyloid typing.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
C.F.d.L. received the “Josep Font” grant from the Hospital Clinic de Barcelona and a travel award from the Spanish Society of Hematology. P.M. and F.L. are partly supported by an investigator fellowship from Fondazione Mintas, Ghislieri College, Pavia, Italy. This work was partly supported by grants from the “Associazione Italiana per la Ricerca sul Cancro” Special Program Molecular Clinical Oncology 5 per mille n. 9965 “Harnessing tumor cell/microenvironment cross talk to treat mature B cell tumors” from the Cariplo Foundation (n 2013-0964), grant RD12/0036/0046 from Instituto de Salud Carlos III (Ministerio de Economía y Competitividad, Spain), and from the Italian Health Ministry to the IRCCS Policlinico San Matteo Foundation, Pavia (grant RC08017800/12).
Authorship
Contribution: C.F.d.L., L.V., G.P., and G.M. conceived and designed the study; L.V., P.M., F.L., A.F., L.O., P.M., G.L.C., and M.P. provided study materials and patients; C.F.d.L., L.V., A.F., L.O., P.M., and G.L.C. collected and assembled the data; C.F.d.L., G.P., C.K., and G.M. analyzed and interpreted the data; C.F.d.L., P.M., G.P., M.P., C.K., and G.M. wrote the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlos Fernández de Larrea, Amyloidosis and Myeloma Unit, Department of Hematology, Hospital Clínic and Institut d’Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS), 08036 Barcelona, Spain; e-mail: cfernan1@clinic.ub.es.