Key Points
Morgana haploinsufficiency in mice causes a lethal and transplantable CML-like myeloid neoplasm.
Morgana is underexpressed in aCML and in a subgroup of CMLs, where it predicts a worse response to imatinib but sensitivity to ROCK inhibitors.
Abstract
We recently described morgana as an essential protein able to regulate centrosome duplication and genomic stability, by inhibiting ROCK. Here we show that morgana+/− mice spontaneously develop a lethal myeloproliferative disease resembling human atypical chronic myeloid leukemia (aCML), preceded by ROCK hyperactivation, centrosome amplification, and cytogenetic abnormalities in the bone marrow (BM). Moreover, we found that morgana is underexpressed in the BM of patients affected by atypical CML, a disorder of poorly understood molecular basis, characterized by nonrecurrent cytogenetic abnormalities. Morgana is also underexpressed in the BM of a portion of patients affected by Philadelphia-positive CML (Ph+ CML) caused by the BCR-ABL oncogene, and in this condition, morgana underexpression predicts a worse response to imatinib, the standard treatment for Ph+ CML. Thus, morgana acts as an oncosuppressor with different modalities: (1) Morgana underexpression induces centrosome amplification and cytogenetic abnormalities, and (2) in Ph+ CML, it synergizes with BCR-ABL signaling, reducing the efficacy of imatinib treatment. Importantly, ROCK inhibition in the BM of patients underexpressing morgana restored the efficacy of imatinib to induce apoptosis, suggesting that ROCK inhibitors, combined with imatinib treatment, can overcome suboptimal responses in patients in which morgana is underexpressed.
Introduction
Chronic myeloid leukemia (CML) is a neoplastic disorder of the hematopoietic stem cells that accounts for 15% to 20% of newly diagnosed cases of adult leukemia. The causative molecular event in CML is the genomic reciprocal translocation t(9;22)(q34;q11), known as Philadelphia (Ph) chromosome. The consequence of this genomic rearrangement is the formation of the BCR–ABL fusion gene, resulting in the production of a chimeric protein with aberrant tyrosine kinase activity. Inhibition of BCR-ABL kinase activity through tyrosine kinase inhibitors (TKI) treatment is successfully used in Ph+ CML to induce remission from the pathology. However, treatment with TKI does not completely eradicate CML because of the insensitivity of CML stem cells to those drugs.1-3 Therefore the identification of pathways that can cooperate with BCR-ABL in the development or in the maintenance of CML is mandatory to identify additional targets to achieve synthetic lethality in the aim of curing CML. Furthermore, 5% of CML patients lack BCR-ABL and are affected by a so-called “atypical CML,” a disease with a poorly understood etiology. Recurrent mutations of SETBP1 are described in ∼24% of aCML.4 Because of the absence of BCR-ABL, aCML patients are not eligible for imatinib treatment, and therefore the identification of specific oncogenic mechanisms is essential to develop a molecular therapy. Typically, aCML is characterized by a high incidence of nonrecurrent cytogenetic abnormalities, often because of aneuploid karyotypes,5-7 suggesting that genes involved in maintenance of genomic stability could be involved in this pathogenesis.
We recently characterized morgana/chp-1 as an essential protein in mouse embryonic development, involved in the regulation of centrosome duplication and genomic stability.8 In fact, morgana+/– mouse embryonic fibroblasts (MEFs), expressing 50% of the normal morgana level, show a higher frequency of supernumerary centrosomes, multipolar spindles, and aneuploid and polyploid karyotypes.8 At a molecular level, morgana forms a complex with ROCKI and ROCKII8 and, by inhibiting ROCKII kinase activity, suppresses centrosome overduplication.8-10 Supernumerary centrosomes represent an important characteristic in cancer onset and progression11 leading to multipolar mitosis, genomic instability,12 and chromothripsis.13 Accordingly, morgana+/– MEFs acquire transformed features over time in vitro.8 In addition, morgana+/– mice show increased susceptibility to tumor development in response to chemical mutagens.8 Here we show that morgana+/– mice develop with age a fatal and transplantable myeloproliferative disease similar to human aCML, presenting centrosome amplification as well as cytogenetic abnormalities in the bone marrow (BM). Notably, low morgana expression levels were found in the BM of aCML-affected patients and in a portion of Ph+ CML. In this latter condition, low morgana levels cooperate with BCR-ABL oncogenic signal in promoting ROCK activity, reducing sensitivity to imatinib treatment.
Methods
Mice
The morgana gene (chordc1) was inactivated by homologous recombination in mouse ES cells as described.8 The genetic background of the morgana+/– and the wild-type mice analyzed was C57BL/6 × 129SV. The use of animals was in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, and was approved by the Animal Care and Use Committee of the University of Torino.
Immunohistochemistry
Immunohistochemistry experiments were performed on formalin-fixed, paraffin-embedded tissues using the following antibodies: antimyeloperoxidase (ab45977, Abcam, 1 μg/mL), anti-P-MLC2 (LS-C16676, LifeSpan Biosciences), and anti-morgana P1/PP0 antibody (10 μg/mL).8,14 Morgana expression level was defined as low when staining intensity was lower compared with normal BM, specifically in the myeloid progenitor cells. Slides were scored independently by 2 pathologists. Furthermore, immunohistochemistry (IHC) scoring was also performed using IHC Profiler software.15
Flow cytometry
Hematopoietic cells were obtained from mouse BM, peripheral blood, and spleen. After lysis of red blood cells, cells were stained with the indicated antibodies (see supplemental Methods). For each analysis, a total of at least 10 000 cells were analyzed. For intracellular morgana staining Lin+ and Lin– BM cells were incubated with antibodies and treated with FIX & PERM (Caltag). Morgana was stained using FITC-conjugated P1/PP0 (5 µg/mL). Flow cytometric analyses were carried out on a FACSCalibur using CellQuest Software (Becton Dickinson).
Bone marrow transplantation
Sublethal total body irradiation, at a dose of 5 Gy, was performed in nude mice. Lin– BM cells were purified using Lineage Cell Depletion Kit for mouse (Miltenyi Biotec) from BM cells obtained from the femurs and tibias of morgana+/– and wild-type littermates. Next, 1 × 106 Lin– cells were injected into the tail veins of irradiated nude mice.
BM transduction
Colony assay
2 × 104 mouse BM cells were plated on M3434 semisolid methylcellulose medium (StemCell Technologies) and scored 12 to 14 days later. For serial replating assays, primary colonies were recovered from the methylcellulose medium. Cells were replated in M3434 media followed by serial replating every 8 to 10 days.
Immunofluorescence
BM cells were fixed in phosphate-buffered saline 4% paraformaldehyde after cytospin and stained with anti-γ-tubulin (T5192 Sigma), anti-α-tubulin (ab15246, Abcam), or anti-morgana (P1/PP0; 10 μg/mL). Immunocomplexes were detected with anti-rabbit IgG Alexa Fluor 488 (A11008, Invitrogen), and anti-mouse IgG Alexa Fluor 568 and 488 (A11004, Invitrogen). Cells were visualized with Apotome software (Zeiss).
Western blot analysis
BM cells were washed in cold phosphate-buffered saline and lysed in a buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM ethylene diamine tetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 1% Triton X-100, 1 mM β-glycerolphosphate, and 1 mM orthovanadate and Protease Inhibitor Cocktail (Sigma). Western blot analysis was performed as described14 with the indicated antibodies (see supplemental Methods).
Cell lines and morgana silencing
The K562 cell line was purchased from American Type Culture Collection (ATCC). Morgana knockdown was performed by infecting K562 cells with pGIPZ lentiviral particles expressing turboGFP and 2 different shRNAs targeting morgana (Open Biosystems).14
Patient BM samples
Primary leukemia cells were obtained from the BM of patients with myeloproliferative disorders (chronic-phase aCML and Ph+ CML) after appropriate informed consent and Institutional Review Board approval (Institutional Ethics Committee Approval # 81/2011). All samples were collected at diagnosis before initiation of treatment.
K562 and BM cell treatment
K562 and patient BM cells were cultured in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in 5% CO2. BM cells were treated for 96 hours with imatinib (10 μM) or fasudil (10 μM), or a combination of the 2 drugs. K562 were cultured for 48 hours with imatinib (1 μM), fasudil (10 μM), Y27632 (20 μM), or a combination of all of them. Effective concentration (IC50) values were calculated by regression analysis using the dose-response curves generated from the experimental data, using GraphPad PRISM 5 Software. Mouse BM cells were cultured in Dulbecco’s modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in 5% CO2 in the presence of interleukin-3 (6 ng/mL; 213-13, Peprotech), interleukin-6 (2.5 ng/mL; 216-16, Peprotech), and stem cell factor (50 ng/mL; 250-03, Peprotech) for 48 hours in presence of fasudil (10 μM), LY294002 (Calbiochem), and BEZ235 (Chemdea).
Statistics
Two-sided Student t test and 1-way analysis of variance were calculated using GraphPad Prism software. P values < .05 were considered statistically significant. Data presented with column graphs and error bars represent the average ± standard error.
Results
Morgana haploinsufficiency causes a myeloproliferative neoplasm in mice
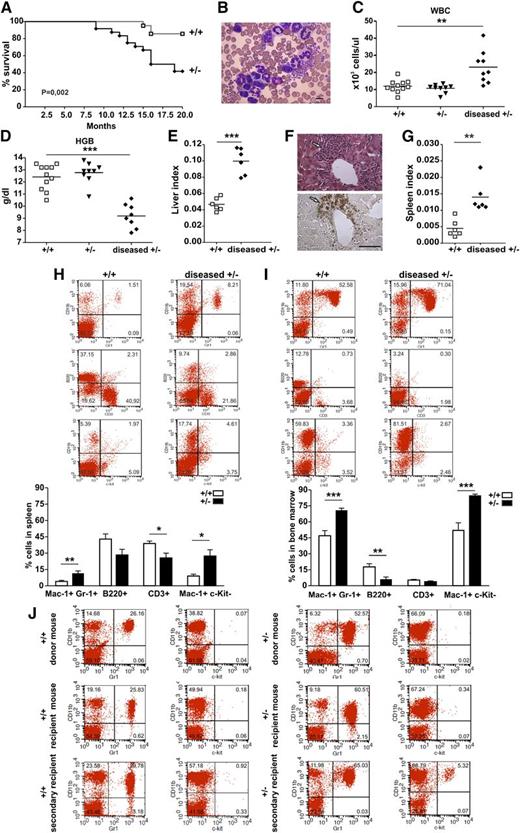
We assessed the susceptibility of morgana+/– mice to spontaneous tumor formation by monitoring them for 20 months. From 12 months of age onward, 60% of morgana+/– mice showed clear pathologic signs such as rapid weight loss, hunched posture, and severe hypokinesia and died as a result (Figure 1A). Necroscopic examination did not reveal the presence of solid tumors, but histopathologic analysis disclosed the presence of myeloid infiltrates in different organs. morgana+/– and wild-type littermates were therefore subjected every 2 months to peripheral blood analysis and, where overt pathologic signs were evident, they were considered moribund, killed, and subjected to blood and hematopoietic organ examinations. morgana+/– moribund mice showed the presence of immature myeloid cells in peripheral blood (Figure 1B) and clear signs of leukocytosis, anemia, anisocytosis, and circulating nucleated red blood cells, whereas no abnormalities were detected in healthy morgana+/– or wild-type mice (Figure 1C-D and supplemental Figure 1A). Notably, expansion of monocytes and eosinophils was not observed (supplemental Figure 1B), whereas, as reported in other myeloproliferative disorder (MPD) murine models,18 an increase of reticulocytes was present (supplemental Figure 1C-E). morgana+/–-diseased mice also showed hepatomegaly (Figure 1E and supplemental Figure 1F), and in 50% of cases, the presence of myeloid infiltrations in the liver, as demonstrated by positive myeloperoxidase staining (Figure 1F). Moreover, they invariably showed splenomegaly (Figure 1G and supplemental Figure 1G) and a significant increase of Mac-1+ Gr-1+ and Mac-1+ c-Kit– cells, paralleled by a reduction in CD3+ cells in the spleen, compared with control mice (Figure 1H). BM of morgana+/–-diseased mice showed a strong expansion of Mac-1+ Gr-1+ and Mac-1+ c-Kit– cells and a reduction of B220+ cells when compared with wild-type mice, demonstrating the expansion of the myeloid population to the detriment of the lymphoid compartment (Figure 1I). Interestingly, in 2 morgana+/–-diseased mice, we detected the presence of a pool of Mac-1– c-Kit+ cells in peripheral blood, suggesting that the myeloproliferative disease was evolving into blast crisis (supplemental Figure 1H).
Evaluation of the spontaneous onset of hematopoietic neoplasm in morgana+/– mice. (A) Survival curves of morgana+/+ and morgana+/– mice. Kaplan-Meier analysis demonstrates a significant difference in survival (morgana+/+: n = 21 and morgana+/–: n = 25). (B) Representative picture of peripheral blood smears of diseased morgana+/– mice. The scale bar = 10 μm. (C-D) White blood cell (WBC) count and hemoglobin (HGB) values in the peripheral blood of diseased morgana+/– mice compared with healthy morgana+/– and wild-type littermates. (E) Liver/body weight ratios (liver index) of morgana+/–-diseased and wild-type mice. (F) Myeloid infiltration in the liver of morgana+/–-diseased mice as confirmed by myeloperoxidase staining. The scale bar = 100 μm. (G) Spleen/body weight ratios (spleen index) of morgana+/–-diseased and wild-type mice. (H-I) Flow cytometric analysis of (H) spleen and (I) BM single-cell suspension from diseased morgana+/– and wild-type littermates and relative quantifications (n = 6 mice per group). (J) Flow cytometric analysis on the peripheral blood of representative morgana+/– and morgana+/+ donor, primary, and secondary recipient mice are shown. *P < .05, **P < .01, ***P < .001.
Evaluation of the spontaneous onset of hematopoietic neoplasm in morgana+/– mice. (A) Survival curves of morgana+/+ and morgana+/– mice. Kaplan-Meier analysis demonstrates a significant difference in survival (morgana+/+: n = 21 and morgana+/–: n = 25). (B) Representative picture of peripheral blood smears of diseased morgana+/– mice. The scale bar = 10 μm. (C-D) White blood cell (WBC) count and hemoglobin (HGB) values in the peripheral blood of diseased morgana+/– mice compared with healthy morgana+/– and wild-type littermates. (E) Liver/body weight ratios (liver index) of morgana+/–-diseased and wild-type mice. (F) Myeloid infiltration in the liver of morgana+/–-diseased mice as confirmed by myeloperoxidase staining. The scale bar = 100 μm. (G) Spleen/body weight ratios (spleen index) of morgana+/–-diseased and wild-type mice. (H-I) Flow cytometric analysis of (H) spleen and (I) BM single-cell suspension from diseased morgana+/– and wild-type littermates and relative quantifications (n = 6 mice per group). (J) Flow cytometric analysis on the peripheral blood of representative morgana+/– and morgana+/+ donor, primary, and secondary recipient mice are shown. *P < .05, **P < .01, ***P < .001.
Finally, to distinguish leukemia from less aggressive disturbances of hematopoiesis, we performed BM transplantation experiments.19 Lineage-negative BM cells were purified from the BM of morgana+/–-diseased mice and wild-type littermates and injected into the tail vein of sublethally-irradiated nude mice. After 3 to 6 months, morgana+/–-recipient mice showed clear pathologic signs, demonstrating that the hematologic neoplasm of morgana+/– mice is transplantable (Figure 1L, supplemental Figure 1I-L, and supplemental Table 1). Notably, in a secondary recipient mouse the pathology progressed toward a more aggressive phenotype, as demonstrated by the presence of a pool of c-Kit+ cells in peripheral blood (Figure 1L), suggesting the clonal evolution of the disorder. Overall, these data indicate that with age, morgana+/– mice develop a fatal and transplantable myeloproliferative disease closely resembling human CML.
To characterize the origin of the disease, we analyzed possible pathologic signs in morgana+/– mice at 8 months. No differences were found in peripheral blood counts (supplemental Figure 2A), in BM subcellular populations (supplemental Figure 2B), or in the presence of dysplastic megakaryocytes in the BM (supplemental Figure 2C). Moreover, morgana+/– and wild-type mice did not differ in hematopoietic stem cell number, basal apoptotic level, proliferation, and P-MLC2 expression levels (supplemental Figure 2D-G). BM cells from healthy morgana+/– mice and wild-type littermates were subjected to a colony assay to assess their clonogenic potential. The frequency of erythroid, granulocytic, and monocytic colonies in morgana+/– mice was comparable with that of wild-type mice (supplemental Figure 2H); however, a slight, but significant, increase in morgana+/– cell-replating potential was noticed (supplemental Figure 2I-L), indicating that morgana+/– cells can acquire hyperproliferative capacity. These observations correlate with the increased proliferation rate of morgana+/– MEFs,8 suggesting that low levels of morgana favor cellular proliferation.
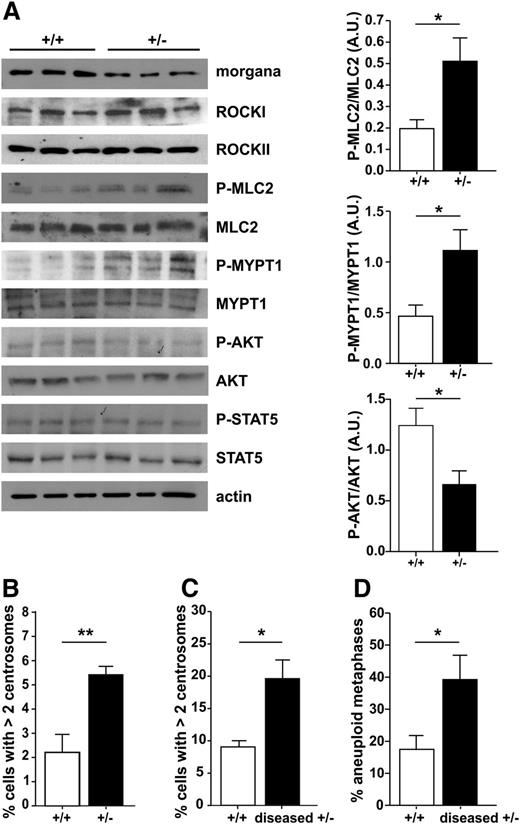
We previously demonstrated that morgana acts as a ROCK inhibitor in primary fibroblasts and that morgana underexpression causes centrosome amplification and genomic instability.8 Analysis of the phosphorylation status of the ROCK substrates MLC-2 and MYPT1 in the BM of morgana+/– mice before disease onset demonstrated that a low morgana expression level enhances ROCK signaling in BM cells (Figure 2A). In addition, AKT phosphorylation is significantly lower in morgana+/– mice (Figure 2A), implying a role for morgana in regulating AKT activation also in BM, other than in breast cancer cells.14 However, morgana+/– BM cells did not show a higher sensitivity to PI3K inhibitors (supplemental Figure 2M). Moreover, morgana+/– mice have a higher number of BM cells with >2 centrosomes when compared with wild-type mice both before (Figure 2B) and after (Figure 2C) disease onset. Notably, in morgana+/–-diseased mice, the number of aberrant metaphases drastically increased (Figure 2D).
Molecular pathogenesis of morgana+/– myeloproliferative disease. (A) Western blot analysis of BM protein extracts from morgana+/+ and morgana+/– mice before disease onset, stained with antibodies against morgana, ROCKI and II, phosphorylated MLC2 (P-MLC2), total MLC2, P-MYPT1, total MYPT1, P-AKT, total AKT, P-STAT5, total STAT5, and actin, used as a loading control. The graphs show the densitometric quantification of P-MLC2, P-MYPT1, and P-AKT bands normalized to total MLC2, MYPT1, and AKT (n = 3 mice per group). Note that ROCKI, ROCKII, and STAT5 phosphorylation were similar in morgana+/+ and morgana+/– BM. (B) Percentages of BM cells with >2 centrosomes in 8-month-old morgana+/+ (n = 6) and morgana+/– (n = 5) mice. (C) Percentages of BM cells with >2 centrosomes in morgana+/–-diseased mice and morgana+/+ littermates (n = 4 mice per group). (D) Percentages of aneuploid metaphase spreads obtained from the BM of morgana+/+ and morgana+/–-diseased mice (n = 4 mice per group). *P < .05, **P < .01.
Molecular pathogenesis of morgana+/– myeloproliferative disease. (A) Western blot analysis of BM protein extracts from morgana+/+ and morgana+/– mice before disease onset, stained with antibodies against morgana, ROCKI and II, phosphorylated MLC2 (P-MLC2), total MLC2, P-MYPT1, total MYPT1, P-AKT, total AKT, P-STAT5, total STAT5, and actin, used as a loading control. The graphs show the densitometric quantification of P-MLC2, P-MYPT1, and P-AKT bands normalized to total MLC2, MYPT1, and AKT (n = 3 mice per group). Note that ROCKI, ROCKII, and STAT5 phosphorylation were similar in morgana+/+ and morgana+/– BM. (B) Percentages of BM cells with >2 centrosomes in 8-month-old morgana+/+ (n = 6) and morgana+/– (n = 5) mice. (C) Percentages of BM cells with >2 centrosomes in morgana+/–-diseased mice and morgana+/+ littermates (n = 4 mice per group). (D) Percentages of aneuploid metaphase spreads obtained from the BM of morgana+/+ and morgana+/–-diseased mice (n = 4 mice per group). *P < .05, **P < .01.
Morgana is underexpressed in human aCML and in a subgroup of Ph+ CMLs
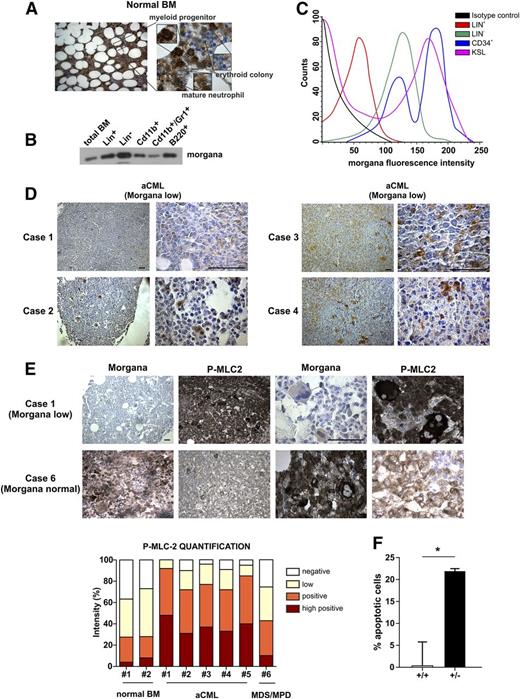
To analyze the relevance of morgana in human pathology, we first evaluated its expression in normal human BM by IHC. This analysis indicated that Morgana is expressed at different levels according to the BM cell type. In particular, Morgana is highly expressed in the myeloid progenitor compartment with a subsequent reduction during differentiation into mature neutrophils. Morgana is also highly expressed in megakaryocytes, whereas mature erythroid colonies and red blood cells show undetectable expression (Figure 3A). Similar results were obtained in mouse BM, where Western blot and fluorescence-activated cell sorting analyses confirmed high levels of morgana expression in progenitor and staminal BM cells (Figure 3B-C).
Morgana is underexpressed in aCML patients’ BM. (A) Morgana IHC staining on normal human BM. (B) Western blot analysis of morgana in different BM populations and in total mouse BM. (C) Morgana fluorescence intensity in different mouse BM populations as assessed by flow cytometry. (D) Morgana IHC staining on BM biopsies of 4 aCML patients. The scale bar = 100 μm. (E) P-MLC2 IHC staining on a BM biopsy from an aCML patient expressing low morgana level (case 1) and a case of MDS/MPD expressing normal morgana level (case 6). The scale bar = 100 μm. The graph shows the IHC quantification of P-MLC2. (F) Percentages of apoptosis in BM cells derived from morgana+/+ and morgana+/–-diseased mice after 48 hours of treatment with fasudil (10 μM). *P < .05.
Morgana is underexpressed in aCML patients’ BM. (A) Morgana IHC staining on normal human BM. (B) Western blot analysis of morgana in different BM populations and in total mouse BM. (C) Morgana fluorescence intensity in different mouse BM populations as assessed by flow cytometry. (D) Morgana IHC staining on BM biopsies of 4 aCML patients. The scale bar = 100 μm. (E) P-MLC2 IHC staining on a BM biopsy from an aCML patient expressing low morgana level (case 1) and a case of MDS/MPD expressing normal morgana level (case 6). The scale bar = 100 μm. The graph shows the IHC quantification of P-MLC2. (F) Percentages of apoptosis in BM cells derived from morgana+/+ and morgana+/–-diseased mice after 48 hours of treatment with fasudil (10 μM). *P < .05.
The phenotype observed in morgana+/– mice, resembling human CML, prompted us to investigate morgana levels in human CML and aCML. Both diseases are myeloproliferative disorders with similar clinical manifestations. Although the hallmark of CML is the Ph chromosome, coding for the chimeric protein BCR-ABL, aCML lacks the expression of this fusion product. Given that BCR-ABL translocation does not occur in mice20 and that the myeloproliferative disease affecting morgana+/– mice strongly resembles CML, we decided to analyze morgana expression levels by IHC in 5 BM samples from aCML patients and 1 case of the closely related myelodysplastic/myeloproliferative neoplasm (MDS/MPN). Strikingly, all 5 aCML samples tested expressed a very low/undetectable morgana level (Morgana low) in the myeloid compartment, whereas the MDS/MPN sample showed normal morgana expression (Figure 3D). Notably, P-MLC2 detection by IHC was inversely correlated with morgana expression levels in BM from aCML patients compared with the MDS/MPN (case 6) (Figure 3E). These observations together with morgana+/– mouse phenotype, strongly suggests that morgana plays a role in human aCML pathogenesis. Interestingly, CSF3R mutations were absent in all samples tested, whereas SETPB1 was found mutated in 1 aCML patient. To assess whether morgana gene (CHORDC1) mutations can be responsible for low morgana expression levels in aCML patients, we screened exome-sequencing data obtained from 16 aCML patients.4 However, no mutations were detectable, suggesting that more complex regulatory mechanisms are involved in morgana underexpression in this pathology.
To evaluate the possibility that Morgana low leukemia cells were addicted to ROCK signaling, we treated BM from morgana+/+- and morgana+/–-diseased mice with fasudil (10 μM) for 48 hours and we analyzed cell viability. ROCK inhibition promoted apoptosis induction only in morgana+/– BM (Figure 3F), suggesting that ROCK inhibitors may have a therapeutic value in aCML.
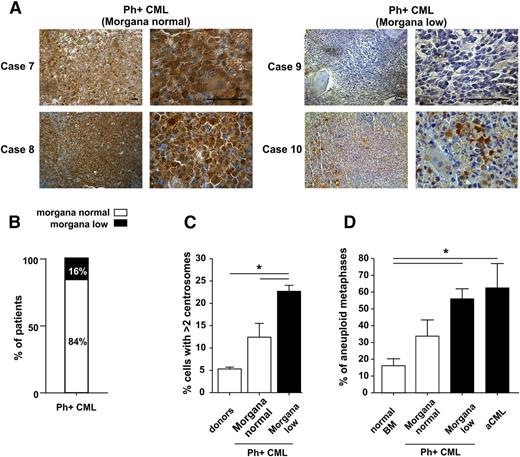
Given that oncosuppressor downregulation often cooperates with activated oncogenes in tumor growth and progression, we expanded our analysis to Ph+ CML. IHC analysis on 19 BM patients revealed that morgana was clearly underexpressed in 16% of patients (Figure 4A-B and supplemental Figure 3A-B). These data were also confirmed by immunofluorescence on CD34+-purified cells (supplemental Figure 3C).
Morgana is underexpressed in a subgroup of CML Ph+ patients. (A) Morgana IHC staining on BM biopsies of representative Morgana normal (cases 7 and 8) and Morgana low (cases 9 and 10) Ph+ CML patients. The scale bar = 100 μm. (B) The graph indicates the percentage of Ph+ CML patients expressing morgana at normal (84%) and low (16%) levels as assessed by IHC analysis on BM sections (n = 19). (C) Percentages of CD34+ BM cells with >2 centrosomes in CML patients at diagnosis, expressing normal or low morgana levels (n = 4 patients per group) and healthy donors (n = 3). The black bar indicates low morgana expression levels. (D) Percentage of aneuploid metaphases in Morgana normal and Morgana low Ph+ CML and Morgana low aCML BM cells compared with normal BM cells. The black bars indicate low morgana expression levels. *P < .05.
Morgana is underexpressed in a subgroup of CML Ph+ patients. (A) Morgana IHC staining on BM biopsies of representative Morgana normal (cases 7 and 8) and Morgana low (cases 9 and 10) Ph+ CML patients. The scale bar = 100 μm. (B) The graph indicates the percentage of Ph+ CML patients expressing morgana at normal (84%) and low (16%) levels as assessed by IHC analysis on BM sections (n = 19). (C) Percentages of CD34+ BM cells with >2 centrosomes in CML patients at diagnosis, expressing normal or low morgana levels (n = 4 patients per group) and healthy donors (n = 3). The black bar indicates low morgana expression levels. (D) Percentage of aneuploid metaphases in Morgana normal and Morgana low Ph+ CML and Morgana low aCML BM cells compared with normal BM cells. The black bars indicate low morgana expression levels. *P < .05.
Given that morgana+/–-diseased mice showed centrosome amplification in BM cells, we assessed whether Morgana low Ph+ CML patients showed similar features. It is known that BCR-ABL expression alone induces centrosome amplification (see also Figure 4C).21-23 However, immunofluorescence analysis of CD34+-purified cells from the BM of Ph+ CML patients highlighted the presence of a significantly higher number of cells with supernumerary centrosomes in Morgana low patients (Figure 4C).
Given that centrosome amplification causes aneuploidy, we analyzed metaphases from Ph+ CML and aCML collected at the moment of diagnosis, and we observed a correlation between morgana underexpression and the percentage of aneuploid cells. In particular, BM of Morgana low patients showed the highest levels of aneuploidy (Figure 4D).
Low morgana in Ph+ CML predicts a suboptimal response to imatinib that can be rescued by inhibiting ROCK
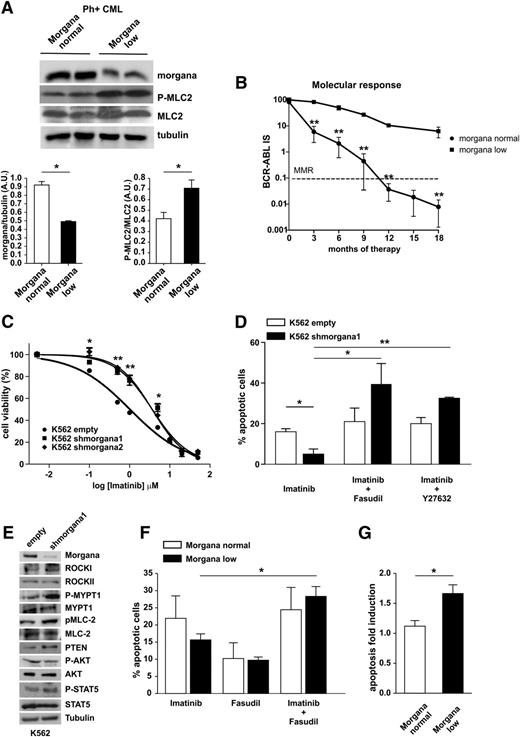
Imatinib blocks the activity of BCR-ABL and of its downstream effector ROCK, leading to CML cell apoptosis.24-27 Given that low morgana levels lead to ROCK activation also in Ph+ CML BM cells (Figure 5A and supplemental Figure 3D), we reasoned that morgana underexpression, by sustaining ROCK activity, may cause a suboptimal response to imatinib treatment. Indeed, as is shown in Figure 5B, our follow-up analysis in a cohort of 12 Ph+ CML patients without BCR-ABL mutations affecting imatinib resistance highlighted a significant correlation between morgana underexpression and a suboptimal response to imatinib (detected by patients’ molecular response). Strikingly, a Morgana low CML patient progressed into blast phase in 4 months after the diagnosis. Furthermore, another Morgana low patient displayed Ph chromosome in 13% of metaphases after 18 months of treatment. Real-time analysis on 102 Ph+ CML patients indicated that 30% of them expressed morgana at less than half of the mean of morgana mRNA levels in normal BM (supplemental Figure 4A-B). The follow-up analysis on 22 patients confirmed that Morgana low patients displayed a suboptimal response to imatinib (supplemental Figure 4C).
Morgana underexpression synergizes with BCR-ABL and negatively affects CML treatment efficacy. (A) Western blot analysis of BM protein extracts from 2 representative Morgana normal and Morgana low Ph+ CML patients stained with antibodies against morgana, phosphorylated MLC2 (P-MLC2), total MLC2, and tubulin. The graphs show the densitometric quantification of morgana bands normalized to tubulin and P-MLC2 bands normalized to total MLC2 (n = 4 patients per group). (B) Correlation between normal and low morgana expression levels and patients’ molecular response during TKI treatment in CML patients stratified for the levels of expression of morgana by IHC (Morgana normal, n = 9; Morgana low, n = 3). (C) Dose-response curves of K562 infected with an empty vector (empty) or with vectors carrying 2 different shRNAs against morgana (shmorgana1 and 2) treated for 48 hours with different imatinib concentrations (0.005-0.1-0.5-1-5-10-20-50 μM) analyzed by MTT assay (mean r2 = 0.98). Viability was expressed as a percentage of untreated cells. (D) Percentages of apoptotic K562 empty or shmorgana1 in response to 48 hours’ treatment with imatinib (1 μM), imatinib (1 μM) + fasudil (10 μM), and imatinib (1 μM) + Y27632 (20 μM). (E) Western blot analysis of K562 empty and shmorgana stained with antibodies against morgana, ROCKI, ROCKII, P-MYPT1, MYPT1, P-MLC2, MLC2, PTEN, P-AKT, AKT, P-STAT5, STAT5, and tubulin. (F) Apoptosis quantification of BM cells from Ph+ patients cultured in the presence of imatinib (10 μM), the ROCK inhibitor fasudil (10 μM), or a combination of the 2 drugs (Morgana normal, 6 patients; Morgana low, 9 patients). Bars represent the percentages of Annexin V–positive cells for each treatment. (G) Mean of apoptosis fold-induction calculated on BM cells treated with imatinib and fasudil vs imatinib alone for each individual patient. *P < .05, **P < .01, ***P < .001.
Morgana underexpression synergizes with BCR-ABL and negatively affects CML treatment efficacy. (A) Western blot analysis of BM protein extracts from 2 representative Morgana normal and Morgana low Ph+ CML patients stained with antibodies against morgana, phosphorylated MLC2 (P-MLC2), total MLC2, and tubulin. The graphs show the densitometric quantification of morgana bands normalized to tubulin and P-MLC2 bands normalized to total MLC2 (n = 4 patients per group). (B) Correlation between normal and low morgana expression levels and patients’ molecular response during TKI treatment in CML patients stratified for the levels of expression of morgana by IHC (Morgana normal, n = 9; Morgana low, n = 3). (C) Dose-response curves of K562 infected with an empty vector (empty) or with vectors carrying 2 different shRNAs against morgana (shmorgana1 and 2) treated for 48 hours with different imatinib concentrations (0.005-0.1-0.5-1-5-10-20-50 μM) analyzed by MTT assay (mean r2 = 0.98). Viability was expressed as a percentage of untreated cells. (D) Percentages of apoptotic K562 empty or shmorgana1 in response to 48 hours’ treatment with imatinib (1 μM), imatinib (1 μM) + fasudil (10 μM), and imatinib (1 μM) + Y27632 (20 μM). (E) Western blot analysis of K562 empty and shmorgana stained with antibodies against morgana, ROCKI, ROCKII, P-MYPT1, MYPT1, P-MLC2, MLC2, PTEN, P-AKT, AKT, P-STAT5, STAT5, and tubulin. (F) Apoptosis quantification of BM cells from Ph+ patients cultured in the presence of imatinib (10 μM), the ROCK inhibitor fasudil (10 μM), or a combination of the 2 drugs (Morgana normal, 6 patients; Morgana low, 9 patients). Bars represent the percentages of Annexin V–positive cells for each treatment. (G) Mean of apoptosis fold-induction calculated on BM cells treated with imatinib and fasudil vs imatinib alone for each individual patient. *P < .05, **P < .01, ***P < .001.
These data suggest that low morgana levels can predict response to imatinib treatment. These observations led us to investigate therapeutic implications in Morgana low CML.
Mali et al demonstrated recently that BCR-ABL causes a constitutive activation of ROCK that sustains cell transformation and survival.24 We then tested whether low morgana level affects the response to imatinib by sustaining ROCK activity. First, we interfered morgana in K562 cells and tested their response to imatinib treatment by determining their dose-response curves (Figure 5C). IC50 value for K562 empty was 1.0 μM, and for K562 silenced for morgana was 3.65 μM (shmorgana1) and 3.63 μM (shmorgana2). Moreover, cells downregulated for morgana showed a reduced apoptotic response to imatinib (Figure 5D). However, when cells were treated with a combination of imatinib and the ROCK inhibitors fasudil or Y27632, the apoptotic response was restored to normal levels (Figure 5D). Signaling analysis demonstrated that morgana downregulation promotes ROCK activation also in the K562 CML cell line, as demonstrated by increased MLC2 and MYPT1 phosphorylation (Figure 5E). It has been reported that ROCKI can phosphorylate PTEN, increasing its stability and activity.28,29 Moreover, we recently demonstrated that morgana overexpression, by inhibiting ROCK kinase activity, results in reduced PTEN levels.14 Accordingly, in Morgana low K562, PTEN was expressed at higher levels. However, AKT phosphorylation was not significantly lower, likely a result of BCR-ABL–dependent PI3K hyperactivation (Figure 5E). STAT5 has been involved in imatinib sensitivity.30 However, STAT5 phosphorylation, albeit showing a slight upregulation in K562 interfered for morgana, was not significantly different (Figure 5E).
Moreover, combined treatment with imatinib and the ROCK inhibitor fasudil significantly increased apoptosis in Morgana low Ph+ CML BM cells (Figure 5F). To eliminate the variability caused by the heterogeneity of response to imatinib in cells of different patients, we calculated the apoptosis fold induction of cells treated with imatinib and fasudil vs imatinib alone for each individual patient, and we observed a significant increase in the percentage of apoptosis in Morgana low compared with BM cells expressing a normal level of morgana (Figure 5G).
However, as for BCR-ABL addiction, morgana haploinsufficiency could not be exploited to eradicate leukemia stem cells. In fact, the apoptotic response to fasudil was not significantly different in BCR-ABL KSL derived from morgana+/– vs wild-type mice (supplemental Figure 3E-G).
Overall, these data indicate that morgana underexpression, through ROCK hyperactivation, causes a suboptimal response to TKI in Ph+ CML cells that can be rescued by ROCK inhibitors, pointing to a potential efficacy of ROCK inhibitors in the chronic treatment of Ph+ CML.
Discussion
Here we show that morgana, a chaperone protein31-35 regulating ROCK activity,8,10,14 is a tumor-suppressor gene involved in myeloid leukemogenesis. In particular, morgana+/– mice spontaneously develop a fatal Ph-negative CML-like myeloproliferative disease. This disease is indeed characterized by dramatic expansion of myeloid compartment, <2% of basophils, <10% of monocytes, <20% of blasts, anemia, splenomegaly, and the absence of fibrosis in the BM. This disease was transplantable into nude mice and eventually able to evolve into acute leukemia. Murine CML-like MPD is associated with centrosome amplification and cytogenetic abnormalities in the BM. Morgana haploinsufficiency in BM cells leads to ROCK hyperactivation and centrosome amplification. Supernumerary centrosomes cause aneuploidy and chromotripsis, which ultimately lead to tumor onset and progression.13,36,37 Accordingly, BM cells from diseased morgana+/– mice showed a high level of aneuploidy. The phenotype of morgana+/– mice prompted us to investigate whether morgana is involved in human Ph-positive CML and atypical CML. aCML has been proposed to represent a clinically distinct entity from the unclassifiable MDS/MPN, for which the identification of specific markers are needed to better classify, stratify, and treat patients with myeloid disorders.38 aCML shares with Ph+ CML a similar clinical presentation but lacks BCR-ABL and therefore cannot benefit from TKI-specific targeted therapy. The only therapeutic option for these patients is the treatment with conventional cytoreductive drugs. aCML is characterized by somatic mutations at low recurrence of several different genes (TET2, CBL, EZH2, SETBP1, CSF3R, ETNK1, etc)4,39-44 and a high incidence of nonrecurrent cytogenetic abnormalities, often due to aneuploid karyotypes,5-7 suggesting that genes that regulate genomic stability maintenance could be involved in the pathogenesis of aCML. Here we show that morgana is underexpressed in the BM of all aCML patients tested, suggesting that morgana underexpression could represent a common feature in aCML. Given that Morgana underexpression correlates with centrosome amplification and aneuploidy, it is tempting to speculate that morgana plays a role in the onset of this disease in patients. Given that ROCK is hyperactivated in Morgana low aCML BM, ROCK inhibitors, already in clinical use for other applications, could represent a new potential therapeutic approach in aCML. Accordingly, fasudil treatment of BM cells induces an apoptotic response only in morgana+/–-diseased mice, suggesting the dependence of these cells to ROCK signaling.
Further, we show that morgana underexpression in Ph+ CMLs synergizes with BCR-ABL signaling in inducing centrosome amplification and genomic instability. It is known that BCR-ABL fusion protein activates different signaling molecules,45,46 including ROCK,24 and that BCR-ABL–expressing cells show addiction to BCR-ABL27 and ROCK signaling.24-26 Indeed, inhibition of BCR-ABL kinase activity through imatinib treatment is successfully used in Ph+ CML to induce apoptosis in BCR-ABL–expressing cells. However, although the majority of Ph+ CML patients show a good response to imatinib, ∼25% to 30% show insufficient therapeutic effect.47,48 Our follow-up analysis demonstrated that Morgana low patients show a worse response during imatinib treatment. Notably, suboptimal response is highly predictive of worse overall survival.49 Thus, the identification of morgana expression level at the onset of the disease may represent an important prognostic tool to predict a suboptimal response to imatinib. Given that morgana underexpression confers imatinib resistance via ROCK hyperactivation, the combined treatment with Imatinib and a ROCK inhibitor restored a normal apoptotic response in Morgana low Ph+ CML cells, suggesting a new potential therapeutic treatment.
In conclusion, our data show that morgana behaves as a tumor suppressor in the pathogenesis of both aCML and Ph+ CML, via its ability to regulate ROCK activity. Morgana underexpression, promoting ROCK hyperactivation, can have a dual action: it favors genomic instability and simultaneously sustains cell survival and proliferation,24 further resulting in myeloproliferation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Enrico Gottardi and Francesca Crasto for human RNA preparations; Carmen Fava for clinical discussion on CML patients; Tiziana Cravero, Serena Vitale, and Flora D’Anna for skillful technical assistance; Flavio Cristofani and Antonellisa Sgarra for assistance in animal experiments; and Radhika Srinivasan for comments on the manuscript.
This work was supported by AIRC grant IG 11456 AIRC 2011 (M.B.), the Italian Ministry of Health (Giovani Ricercatori–Ricerca Finalizzata 2010; GR-2010-2312984) (A.M.), and a fellowship from AIRC (annual fellowship “Marco Fabio Sartori”) (A.D.).
Authorship
Contribution: A.D.S., S.R., F.F., R.F., C.P., S.C., G.C., A.C., and I.F. performed experiments; U.F. and M.P. performed and supervised the histopathological analysis and immunohistochemistry; E.G. performed cytogenetic analysis on human BM samples; B.M. performed mouse blood examinations; J.C.C. and A.M. performed mice histopathological examination; R.P. and C.G.-P. analyzed exome sequencing data; E.T., L.S., and E.H. provided discussions and advice; P.P.P. and G.T. contributed to experimental design and critical analysis of the results; G.S. and G.R.-C. provided patient samples and critical analysis of data; and A.M. and M.B. designed the research, coordinated and directed the study, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for R.F. is Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA.
Correspondence: Mara Brancaccio, University of Torino, Molecular Biotechnology Center, via Nizza, 52, Torino 10126, Italy; e-mail: mara.brancaccio@unito.it; or Alessandro Morotti, University of Torino, San Luigi Gonzaga Hospital, Orbassano (Torino), Italy; e-mail: alessandro.morotti@unito.it.
References
Author notes
A.D.S., C.P., S.R., A.M., and M.B. contributed equally to this study.