Key Points
ERp5, like its family members PDI and ERp57, accumulates at sites of vessel wall injury.
Both platelets and endothelium secrete ERp5 on activation and contribute ERp5 necessary for thrombus formation in vivo.
Abstract
Protein disulfide isomerase (PDI) and endoplasmic reticulum protein 57 (ERp57) are emerging as important regulators of thrombus formation. Another thiol isomerase, endoplasmic reticulum protein 5 (ERp5), is involved in platelet activation. We show here the involvement of ERp5 in thrombus formation using the mouse laser-injury model of thrombosis and a specific antibody raised against recombinant ERp5. Anti-ERp5 antibody inhibited ERp5-dependent platelet and endothelial cell disulfide reductase activity in vitro. ERp5 release at the thrombus site was detected after infusion of Alexa Fluor 488–labeled anti-ERp5 antibody at 0.05 μg/g body weight, a dose that does not inhibit thrombus formation. Anti-ERp5 at 3 μg/g body weight inhibited laser-induced thrombus formation in vivo by causing a 70% decrease in the deposition of platelets and a 62% decrease in fibrin accumulation compared to infusion of control antibody (P < .01). ERp5 binds to β3 integrin with an equilibrium dissociation constant (KD) of 21 µM, measured by surface plasmon resonance. The cysteine residues in the ERp5 active sites are not required for binding to β3 integrin. These results provide evidence for a novel role of ERp5 in thrombus formation, a function that may be mediated through its association with αIIbβ3.
Introduction
Endoplasmic reticulum protein 5 (ERp5) is a member of a family of thiol isomerases that includes 20 enzymes best recognized for their participation in protein synthesis. The defining characteristic of these enzymes is the presence of thioredoxin-like domains. ERp5 contains 3 thioredoxin-like domains: a, a′, and b. The a and a′ domains contain the active-site motif CXXC, whereas the b domain lacks this motif. Thiol isomerases play an important role during protein synthesis in the endoplasmic reticulum, catalyzing the formation, reduction, or rearrangement of disulfide bonds between cysteine residues.1,2 Increasingly, thiol isomerases have been identified on the surfaces of cells, including platelets, endothelial cells, and lymphocytes.3-8 Although the function of thiol isomerases on the cell surface has not been fully characterized, involvement of oxidoreductase activity has been demonstrated in several cases. Protein disulfide isomerase (PDI)-mediated alteration of disulfide bonds in cell surface proteins has been implicated in the regulation of platelet and neutrophil adhesion,9-12 and PDI’s extracellular catalytic activity is involved in the fusion of HIV to CD4 on lymphocytes.4 The extracellular catalytic activity of ERp5 on the tumor ligand:major histocompatibility complex class-I-related ligand MICA contributes to tumor immunoevasion.13 A number of members of this enzyme family, including the prototypic PDI and endoplasmic reticulum protein 57 (ERp57), are found in platelets and secreted when these cells are activated, and mediate platelet thrombus formation and fibrin generation in mouse models of thrombosis.14-24
Like PDI and ERp57, ERp5 is secreted from platelets on cell activation.25 Inhibition of ERp5 function with an anti-ERp5 antibody prevented fibrinogen binding to activated platelets and platelet aggregation in vitro.25 The fibrinogen receptor αIIbβ3 is a potential substrate of ERp5 because the enzyme coimmunoprecipitates with the β3 chain of the integrin.25 However, an in vivo role for ERp5 in thrombus formation has not been reported. In the current study, we investigated whether ERp5 is released at the site of thrombus formation in vivo and whether inhibition of the ERp5 reductase activity derived from platelets and from endothelium influences platelet thrombus formation and fibrin generation in a laser-induced mouse model of thrombosis.
Materials and methods
The sources for enzymes, antibodies, cells, and assay reagents are identified in supplemental Materials and Methods, available on the Blood Web site. The supplemental material also includes the methods for expression and purification of recombinant ERp5, ERp57, variant ERp5 with the CGHC sequences in the a and a′ domains mutated to AGHA (ERp5-AGHA), and β3 integrin. β3 integrin was expressed with a calmodulin tag to facilitate immunoaffinity purification using conformation-specific antibodies to the calcium ion–stabilized conformer and elution of the β3 integrin with EDTA. β3 integrin was immediately dialyzed into 10 mM HEPES (pH 7.4), 150 mM sodium chloride, 0.005% P20, and 0.5 mM calcium chloride. Wild-type male C57BL/6 mice were from The Jackson Laboratory (Bar Harbor, ME). Mice between 6 and 8 weeks of age were used. All mouse studies were performed with the approval of the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Polyclonal anti-ERp5 antibody
Recombinant human His-tagged ERp5 was used as immunogen to raise polyclonal antibodies in rabbits (Covance, Denver, NJ). Rabbit immunoglobulin (Ig)G was purified from preimmune and immune serum by affinity chromatography using protein A/G-agarose. Anti-ERp5 antibodies were isolated by sequential immunoaffinity chromatography. IgG from immunized rabbits was loaded onto an ERp5/agarose column (ERp5, 3 mg/mL) and bound anti-ERp5 was eluted with glycine buffer, pH 2. Anti-ERp5 IgG dialyzed into phosphate-buffered saline (PBS), pH 7.4, was loaded onto an ERp72/agarose column (ERp72, 2 mg/mL), and the flow-through from this column was loaded onto an ERp57/agarose column (ERp57, 2 mg/mL). The flow-through from this latter column, anti-ERp5 IgG, free of anti-ERp72 and anti-ERp57 cross-reactive IgG, was tested by enzyme-linked immunosorbent assay (ELISA) at concentrations of 0.01, 0.1, and 1 ng/mL for reactivity against recombinant ERp5, ERp72, ERp57, and PDI (coated at 0.1 μg per well of a 96-well plate). The assay was developed with goat anti-rabbit IgG conjugated to horseradish peroxidase (HRP; 0.1 µg/mL) and 50 μL of the HRP chromogenic substrate tetramethylbenzidine (KPL Inc., Gaithersburg, MD).
Dieosin disulfide reductase assay
Dieosin glutathione disulfide, di-E-GSSG, was prepared as previously described.26 Reductase activity of purified enzymes was monitored in 96-well fluorescence microtiter plates. ERp5, ERp5-AGHA, PDI, and ERp57 were assayed at the concentrations indicated in the absence or presence of anti-ERp5 antibody (0.3-2.2 µM). Di-E-GSSG (150 nM) was added to enzyme in the presence of 5 µM dithiothreitol (DTT), and the increase in fluorescence due to release of eosin-glutathione for ERp5, ERp5-AGHA, ERp57, and PDI was determined by excitation at 520 nm and emission at 545 nm in a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). Reductase activity of ERp57 was measured in a Tecan Infinite Pro 200 microplate reader (Tecan Group, Männedorf, Switzerland). The reduction of 150 nM di-E-GSSG by 5 µM DTT alone served as a negative control.
In a modified cellular assay, di-E-GSSG was used to measure cell surface reductase activity.26 Washed platelets (2 ×107) were added to the wells of a 96-well microtiter plate. Anti-ERp5 antibody (0.3-1.2 μM) or control IgG (1.2 μM) was added to the platelet suspension prior to the addition of thrombin (0.5 U/mL), di-E-GSSG (150 nM), and DTT (5 μM). The reduction of di-E-GSSG was monitored as above. The reduction of di-E-GSSG by DTT alone was used as a negative control. In a similar assay, human umbilical vein endothelial cells (HUVECs; 20 × 103) were seeded in the wells of a 96-well microtiter plate overnight and then washed thrice with PBS, pH 7.4. Endothelial cells were incubated with anti-ERp5 antibody (0.3-2.2 μM) or control IgG (2.2 μM) prior to the addition of thrombin (0.5 U/mL), di-E-GSSG (150 nM), and DTT (5 μM).
Denatured RNase disulfide isomerization assay
Isomerase activity of purified ERp5 and PDI was measured by their ability to renature reduced and denatured ribonuclease (RNase).27 Denatured RNase was prepared by suspending 5 mg of RNase A in 1 mL of 100 Tris/acetate (pH 8), 2 mM EDTA, 6 M guanidinium HCl, and 140 mM DTT overnight at 4°C. Immediately prior to use, DTT and guanidinium HCl were removed by gel filtration on PD-10 (GE Healthcare, Buckinghamshire, United Kingdom). Denatured RNase (8 µM) was incubated with 1 mM GSH, 0.2 mM GSSG, and 4.5 mM cCMP in 55 mM Tris/acetate and 1 mM EDTA, pH 8.0, in the presence or absence of purified ERp5 or PDI at 1 µM. Renatured RNase was assayed for its ability to degrade cCMP, measured by the increase in absorbance at 296 nm. Anti-ERp5 antibody (0.3-2.2 µM) was tested for its ability to inhibit renaturation of RNase.
The isomerase assay of ERp57 was modified in accordance with the method of Frickel et al.28 The isomerase activity of ERp57 was tested by its ability to reactivate 40 mM denatured RNase by incubation with 10 μM ERp57 in 50 mM Tris/acetate (pH 8), 2 mM EDTA, and 10 μM DTT in the presence or absence of anti-ERp5 antibody (0.3-2.2 µM) or control IgG (2.2 µM) at 23ο for 8 hours. RNase activity was monitored for cCMP hydrolysis, as above, in a PowerWave X plate reader (BioTek, Winooski ,VT).
Binding of recombinant ERp5 to αIIbβ3
ERp5 was coated overnight at 100 nM in 96-well plates. After blocking, αIIbβ3 with or without 2 mM MnCl2 was added, and the reaction mixtures were incubated for 1 hour at 22°C. Anti-αIIbβ3 antibody (mouse monoclonal VI-PL2, 1 µg/mL) was added, followed by HRP-conjugated donkey anti-mouse IgG (0.1 µg/mL) to detect bound αIIbβ3. Alternatively, full-length native αIIbβ3 or glycoprotein Ibα was coated at 20 nM in a Nunc MaxiSorp 96-well microtiter plate. After blocking, ERp5 or ERp5-AGHA was added at concentrations of 1.0 to 100 nM. Anti-ERp5 antibody or control IgG (1 µg/mL) was added, followed by HRP-conjugated goat anti-rabbit IgG (0.5 μg/mL) to detect bound rabbit IgG.
Surface plasmon resonance
Native αIIbβ3 (50 µg/mL) or recombinant β3 integrin (55 µg/mL) was immobilized on a CM5 Biacore chip. ERp5 was applied at concentrations of 0.33 to 42 µM in running buffer containing 10 mM HEPES (pH 7.4), 150 mM sodium chloride, 0.005% P20, and 0.5 mM calcium chloride with or without 2 mM MnCl2.
Secretion of ERp5 from platelets and endothelial cells
Washed human and mouse platelets were prepared as previously described.29,30 The preparation of lysates and releasates from platelets and endothelial cells, and the subsequent blotting for ERp5 and glyceraldehyde-3-phosphate dehydrogenase and quantitation of ERp5 by ELISA are described in supplemental Materials and Methods.
ERp5 cell surface binding
Mouse platelets (4 × 106) were prepared as above and activated with mouse thrombin at a final concentration of 0.5 U/mL. Resting and activated platelets were incubated with monoclonal anti-human ERp5 antibody or mouse IgG2α,κ, both directly labeled with Alexa Fluor 647 (0.25 mg/mL). Antibody binding was analyzed by flow cytometry.
Effect of anti-ERp5 antibody on platelet aggregation
Mouse platelets were prepared as above and incubated with anti-ERp5 antibody or control IgG at 0.2 μM. Thrombin (0.2 U/mL) was added, and platelet aggregation was monitored over time in a Chrono-log 680 aggregation system.
Intravital microscopy
Laser-induced injury and image analysis
Detection of ERp5 in platelet thrombi in vivo with immunoaffinity-purified anti-ERp5 antibody
Anti-ERp5 or preimmune IgG was labeled with Alexa Fluor 488 (Invitrogen) according to the manufacturer’s instructions. Labeled antibodies were infused into C57BL/6 mice at a dose of 0.05 µg/g body weight, followed by infusion of anti-CD42b antibody labeled with DyLight 649 (0.1 µg/g body weight), and the median intravital fluorescence values from multiple laser-induced thrombi in cremaster arterioles were measured.
Effect of anti-ERp5 antibody on thrombus formation in vivo
Anti-ERp5 antibody (1 and 3 µg/g body weight) or preimmune rabbit control IgG (3 µg/g body weight) in PBS was infused into mice, followed by anti-CD42b antibody labeled with DyLight 649 (0.1 µg/g body weight) and anti-fibrin-specific antibody labeled with Alexa Fluor 488 (0.5 μg/g body weight). Laser-induced platelet thrombus formation and fibrin deposition before and after the infusion of anti-ERp5 antibody or control antibody were measured as the median integrated fluorescence of the anti-platelet and anti-fibrin antibodies over time.
To determine whether ERp5 secreted from endothelial cells is sufficient to support fibrin formation, we measured fibrin generation in eptifibatide-treated mice. Eptifibatide inhibits platelet thrombus formation, but fibrin deposition at sites of laser injury is maintained in eptifibatide-treated animals.17 Fibrin and platelet accumulation were monitored before and after the infusion of eptifibatide alone (10 μg/g initial dose, repeated every 20 minutes during data acquisition) or eptifibatide plus anti-ERp5 antibody at 3 μg/g.
Statistical analysis
The Mann-Whitney test was used for statistical comparison of the areas under the curves for platelet and fibrin fluorescence between 2 groups of animals. Median platelet and fibrin fluorescence curves were derived from 22 to 40 thrombi per group generated in at least 3 mice per group. Statistical analyses were performed using Prism 5.02 software (GraphPad). P values of .05 or less were considered statistically significant and are indicated.
Results
Characterization of recombinant ERp5 and polyclonal anti-ERp5 antibodies
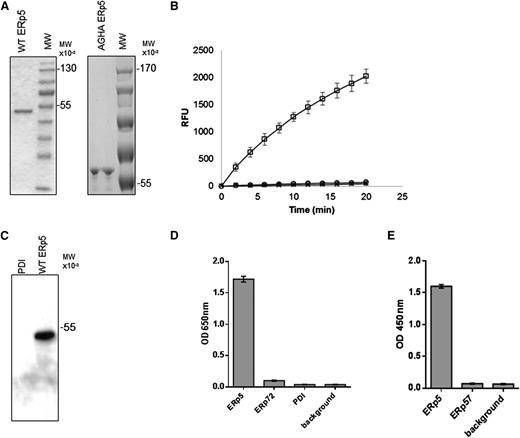
To study the potential role of ERp5 in thrombus formation in vivo, we expressed recombinant ERp5 with an N-terminal His tag and prepared rabbit polyclonal anti-ERp5 antibodies. These antibodies were characterized to assure that there was minimal cross-reactivity with PDI, ERp57, and ERp72. Purified recombinant ERp5 and ERp5-AGHA, a mutated ERp5 with the active-site motif CGHC sequences in the a and a′ domains replaced by AGHA, yielded a single major band on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Figure 1A). We used the di-E-GSSG assay to evaluate the reductase activity of ERp5 and ERp5-AGHA. Reduction of di-E-GSSG with a resulting increase in eosin fluorescence has been used as a sensitive method to detect the reductase activity of PDI, ERp5, and ERp57.21,32 Recombinant ERp5 is catalytically active in the di-E-GSSG reductase assay, whereas ERp5-AGHA does not have reductase activity (Figure 1B).
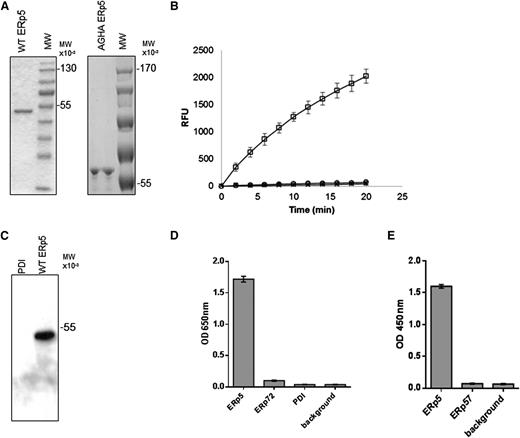
Purification of wild-type ERp5, ERp5-AGHA, and polyclonal antibodies to ERp5. (A) Purified wild-type (WT) ERp5 (2 μg) and ERp5-AGHA (2 μg) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and stained with coomassie blue dye. (B) Disulfide reductase activity of wild-type ERp5 (100 nM; □) and ERp5-AGHA (100 nM; ○). The relative increase in fluorescence produced by the reduction of di-E-GSSG is reported as a function of time. Di-E-GSSG probe plus DTT alone (×) serves as a negative control. (C) Immunoaffinity-purified anti-ERp5 antibody (0.5 μg/mL) detects wild-type ERp5 (50 ng) but does not detect PDI (500 ng) on western blot analysis. (D) ELISA of immunoaffinity-purified anti-ERp5 antibody (0.1 ng/mL) binding to recombinant His-tagged ERp5, His-tagged ERp72, or His-tagged PDI coated at 0.1 μg per well. Background indicates no bound thiol isomerases. N = 3 in triplicate; error bars represent 2 standard deviations. (E) ELISA of immunoaffinity-purified anti-ERp5 antibody (0.1 ng/mL) binding to recombinant His-tagged ERp5 or His-tagged ERp57. Background indicates no bound thiol isomerases. Proteins were coated at 0.1 μg per well. N = 3 in triplicate; error bars represent 2 standard deviations. These assays were developed with goat anti-rabbit IgG conjugated to HRP and to HRP chromogenic substrate tetramethylbenzidine, and OD was measured at 650 nm (D). In some experiments, the tetramethylbenzidine reaction was terminated with the addition of 50 μL of 0.16 M sulfuric acid, and OD was measured at 450 nm (E). MW, molecular weight; OD, optical density; RFU, relative fluorescence units.
Purification of wild-type ERp5, ERp5-AGHA, and polyclonal antibodies to ERp5. (A) Purified wild-type (WT) ERp5 (2 μg) and ERp5-AGHA (2 μg) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and stained with coomassie blue dye. (B) Disulfide reductase activity of wild-type ERp5 (100 nM; □) and ERp5-AGHA (100 nM; ○). The relative increase in fluorescence produced by the reduction of di-E-GSSG is reported as a function of time. Di-E-GSSG probe plus DTT alone (×) serves as a negative control. (C) Immunoaffinity-purified anti-ERp5 antibody (0.5 μg/mL) detects wild-type ERp5 (50 ng) but does not detect PDI (500 ng) on western blot analysis. (D) ELISA of immunoaffinity-purified anti-ERp5 antibody (0.1 ng/mL) binding to recombinant His-tagged ERp5, His-tagged ERp72, or His-tagged PDI coated at 0.1 μg per well. Background indicates no bound thiol isomerases. N = 3 in triplicate; error bars represent 2 standard deviations. (E) ELISA of immunoaffinity-purified anti-ERp5 antibody (0.1 ng/mL) binding to recombinant His-tagged ERp5 or His-tagged ERp57. Background indicates no bound thiol isomerases. Proteins were coated at 0.1 μg per well. N = 3 in triplicate; error bars represent 2 standard deviations. These assays were developed with goat anti-rabbit IgG conjugated to HRP and to HRP chromogenic substrate tetramethylbenzidine, and OD was measured at 650 nm (D). In some experiments, the tetramethylbenzidine reaction was terminated with the addition of 50 μL of 0.16 M sulfuric acid, and OD was measured at 450 nm (E). MW, molecular weight; OD, optical density; RFU, relative fluorescence units.
Immunoaffinity-purified polyclonal anti-ERp5 antibody detected ERp5 on immunoblot but did not detect PDI (Figure 1C). Specificity of the immunoaffinity-purified anti-ERp5 antibody was further evaluated by ELISA, including binding to PDI and ERp72, both secreted from activated platelets.19 The antibodies bound to His-tagged ERp5 but did not exhibit significant cross-reactivity with His-tagged PDI or His-tagged ERp72 compared to background (Figure 1D). Purified anti-ERp5 antibodies bound to His-tagged ERp5 but not to His-tagged ERp57 compared to background (Figure 1E). These results indicate that the affinity-purified anti-ERp5 does not bind measurably to ERp57, ERp72, or PDI.
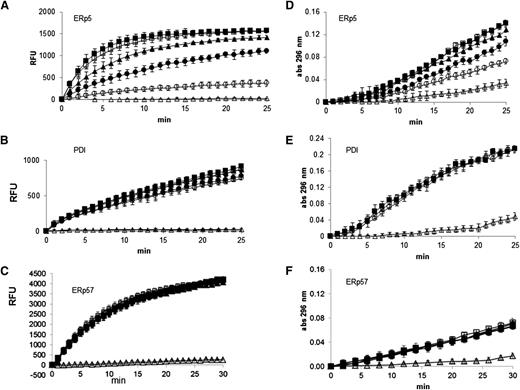
The polyclonal anti-ERp5 antibody inhibits ERp5 reductase activity in a dose-dependent manner (Figure 2A) but only minimally inhibits the reductase activity of His-PDI and does not inhibit His-ERp57 at high concentrations in the di-E-GSSG assay (Figure 2B-C). Anti-ERp5 antibody also inhibited the isomerase activity of ERp5 (Figure 2D) but not that of PDI (Figure 2E) or ERp57 (Figure 2F) in refolding of denatured and reduced RNase. Thus, we used the immunoaffinity-purified anti-ERp5 antibody in experiments to determine whether ERp5 plays a role in thrombus formation in a laser-induced mouse model of thrombosis.
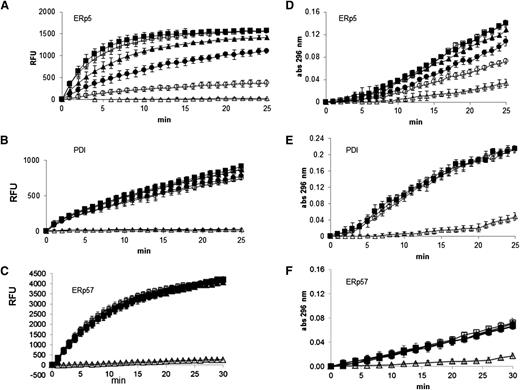
Inhibition of ERp5 disulfide reductase and isomerase activity by anti-ERp5 antibody. (A) ERp5 disulfide reductase activity was measured by reduction of di-E-GSSG as a function of time. The inhibition of ERp5-catalyzed (50 nM) reduction of di-E-GSSG was determined in the presence of increasing amounts of anti-ERp5 antibody. His-PDI (50 nM) reductase activity (B) or His-ERp57 (100 nM) reductase activity (C) was measured in the di-E-GSSG assay in the presence of anti-ERp5 antibody. For panels A-C, anti-ERp5 antibody: none (□), 0.3 µM (▲), 0.6 µM (●), and 2.2 µM (○); preimmune IgG: 2.2 µM (▪); DTT, no enzyme: (△). N = 3 in triplicate. (D) The isomerase activity of ERp5 (1 μM) was measured in the RNase renaturation assay. The inhibition of isomerase activity was measured in the presence of increasing concentrations of anti-ERp5 antibody. (E) The isomerase activity of PDI (1 µM) was measured in the RNase renaturation assay in the absence and presence of anti-ERp5 antibody. (F) The isomerase activity of ERp57 (2.5 µM) was measured in the RNase renaturation assay in the absence and presence of anti-ERp5 antibody. For panels D-F, anti-ERp5 antibody: none (□), 0.3 µM (▲), 0.6 µM (●), and 2.2 µM (○); preimmune IgG: 2.2 µM (▪); denatured and reduced RNase: (△). N = 3 in triplicate. Abs, absorbance.
Inhibition of ERp5 disulfide reductase and isomerase activity by anti-ERp5 antibody. (A) ERp5 disulfide reductase activity was measured by reduction of di-E-GSSG as a function of time. The inhibition of ERp5-catalyzed (50 nM) reduction of di-E-GSSG was determined in the presence of increasing amounts of anti-ERp5 antibody. His-PDI (50 nM) reductase activity (B) or His-ERp57 (100 nM) reductase activity (C) was measured in the di-E-GSSG assay in the presence of anti-ERp5 antibody. For panels A-C, anti-ERp5 antibody: none (□), 0.3 µM (▲), 0.6 µM (●), and 2.2 µM (○); preimmune IgG: 2.2 µM (▪); DTT, no enzyme: (△). N = 3 in triplicate. (D) The isomerase activity of ERp5 (1 μM) was measured in the RNase renaturation assay. The inhibition of isomerase activity was measured in the presence of increasing concentrations of anti-ERp5 antibody. (E) The isomerase activity of PDI (1 µM) was measured in the RNase renaturation assay in the absence and presence of anti-ERp5 antibody. (F) The isomerase activity of ERp57 (2.5 µM) was measured in the RNase renaturation assay in the absence and presence of anti-ERp5 antibody. For panels D-F, anti-ERp5 antibody: none (□), 0.3 µM (▲), 0.6 µM (●), and 2.2 µM (○); preimmune IgG: 2.2 µM (▪); denatured and reduced RNase: (△). N = 3 in triplicate. Abs, absorbance.
ERp5 is secreted from platelets and endothelial cells
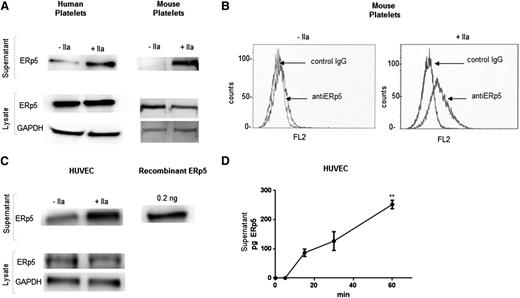
To determine whether ERp5 plays a role in thrombus formation in vivo, we employed our laser-induced mouse model of thrombosis and demonstrated that the immunoaffinity-purified anti-ERp5 antibody raised against human ERp5 binds to mouse ERp5. The polyclonal antibody detected human and mouse ERp5 in lysates and in releasates from human and mouse platelets treated with thrombin (Figure 3A). ERp5 binds to human platelets.19,25 Like human platelets, thrombin-activated mouse platelets bind ERp5 on their surface (Figure 3B). Thrombin-stimulated human platelets release 1.4 fg of ERp5 per platelet. The ERp5 concentration in platelet-poor human plasma was 60 ng/mL.
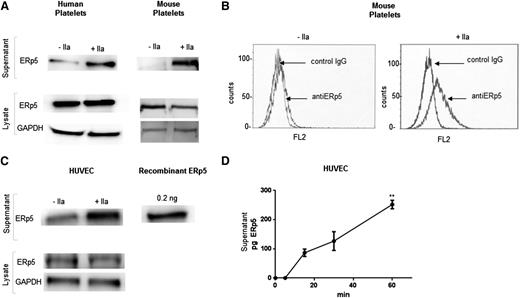
Secretion of ERp5 from platelets and endothelial cells. (A) Detection of ERp5 in lysate and supernatant of human and mouse (C57BL/6) platelets. Equal number of platelets were stimulated with thrombin (+IIa) at a dose of 0.5 U/mL or maintained in the resting state (−IIa). The supernatant (containing the platelet releasate) was separated from the platelets by centrifugation, and the platelets were lysed. Platelet lysates were probed for ERp5 and GAPDH. Platelet supernatants were probed for ERp5. (B) Expression of ERp5 on the surface of mouse platelets. Washed platelets were prepared from C57BL/6 mouse blood and activated with mouse thrombin (0.5 U/mL). Resting (−IIa) and activated (+IIa) platelets were incubated with monoclonal anti-human ERp5 antibody or isotype control (IgG) antibody, both labeled with Alexa Fluor 647. (C) Detection of ERp5 in the supernatant and lysate of cultured HUVECs before (−IIa) and after (+IIa) stimulation with 0.5 U/mL thrombin. HUVEC lysate was probed for ERp5 and GAPDH. HUVEC supernatant was probed for ERp5. (D) Release of ERp5 from thrombin-stimulated HUVECs over time as determined by densitometry compared to ERp5 control. N = 3; error bars represent standard deviation; **P < .005. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pg, picograms.
Secretion of ERp5 from platelets and endothelial cells. (A) Detection of ERp5 in lysate and supernatant of human and mouse (C57BL/6) platelets. Equal number of platelets were stimulated with thrombin (+IIa) at a dose of 0.5 U/mL or maintained in the resting state (−IIa). The supernatant (containing the platelet releasate) was separated from the platelets by centrifugation, and the platelets were lysed. Platelet lysates were probed for ERp5 and GAPDH. Platelet supernatants were probed for ERp5. (B) Expression of ERp5 on the surface of mouse platelets. Washed platelets were prepared from C57BL/6 mouse blood and activated with mouse thrombin (0.5 U/mL). Resting (−IIa) and activated (+IIa) platelets were incubated with monoclonal anti-human ERp5 antibody or isotype control (IgG) antibody, both labeled with Alexa Fluor 647. (C) Detection of ERp5 in the supernatant and lysate of cultured HUVECs before (−IIa) and after (+IIa) stimulation with 0.5 U/mL thrombin. HUVEC lysate was probed for ERp5 and GAPDH. HUVEC supernatant was probed for ERp5. (D) Release of ERp5 from thrombin-stimulated HUVECs over time as determined by densitometry compared to ERp5 control. N = 3; error bars represent standard deviation; **P < .005. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pg, picograms.
ERp5 is constitutively secreted from cultured HUVECs (Figure 3C) and human aortic endothelial cells (data not shown). There is a significant increase in secretion after stimulation with thrombin (Figure 3C-D).
Anti-ERp5 antibody inhibits the reductase activity of platelets and endothelial cells
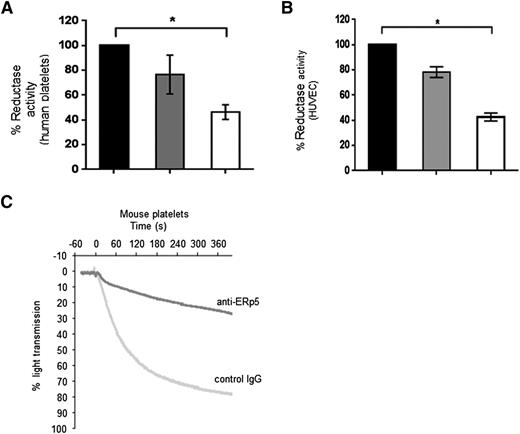
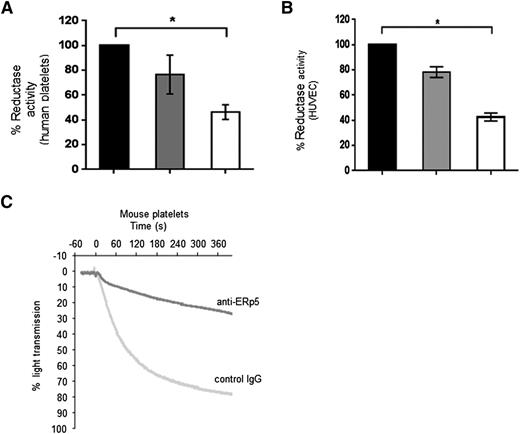
Anti-ERp5 significantly inhibited the reductase activity found at the platelet and endothelial cell surfaces as measured in the di-E-GSSG assay (Figure 4A-B). Our anti-ERp5 antibody also inhibited thrombin-induced aggregation of washed mouse platelets by 75% (Figure 4C), a phenomenon that has been previously observed for human platelets.25
Inhibition of function of platelet and endothelial ERp5 with anti-ERp5 antibody. Inhibition of disulfide reductase activity on the activated cell surface of human platelets and HUVECs measured in the di-E-GSSG reduction assay. (A) Percent reductase activity of platelets in the presence of anti-ERp5 antibody (0.3 µM, gray; 1.2 μM, white) compared to reductase activity of platelets in the presence of control IgG (1.2 μM, black). (B) Percent reductase activity of HUVECs in the presence of anti-ERp5 antibody (0.3 µM, gray; 2.2 μM, white) compared to reductase activity of HUVEC in the presence of control IgG (2.2 μM, black). N = 3 in triplicate; *P < .05. (C) Inhibition of mouse platelet aggregation with anti-ERp5 antibody. Platelets were incubated with control IgG or anti-ERp5 antibody (0.2 μM) and subsequently stimulated with thrombin (0.2 U/mL).
Inhibition of function of platelet and endothelial ERp5 with anti-ERp5 antibody. Inhibition of disulfide reductase activity on the activated cell surface of human platelets and HUVECs measured in the di-E-GSSG reduction assay. (A) Percent reductase activity of platelets in the presence of anti-ERp5 antibody (0.3 µM, gray; 1.2 μM, white) compared to reductase activity of platelets in the presence of control IgG (1.2 μM, black). (B) Percent reductase activity of HUVECs in the presence of anti-ERp5 antibody (0.3 µM, gray; 2.2 μM, white) compared to reductase activity of HUVEC in the presence of control IgG (2.2 μM, black). N = 3 in triplicate; *P < .05. (C) Inhibition of mouse platelet aggregation with anti-ERp5 antibody. Platelets were incubated with control IgG or anti-ERp5 antibody (0.2 μM) and subsequently stimulated with thrombin (0.2 U/mL).
ERp5 is detected at thrombus sites in vivo
Both PDI and ERp57 released from platelets and endothelial cells have been shown to play a role in thrombus formation in vivo in mouse models of thrombosis.16-22 To determine whether ERp5 is also found at sites of thrombus formation, anti-ERp5 antibodies or preimmune IgG labeled with Alexa Fluor 488 (0.05 µg/g body weight) were infused into mice, followed by infusion of a monoclonal anti-CD42b antibody conjugated to DyLight 649 to image platelets. At this dose of anti-ERp5 antibody, release of ERp5 was detected at the thrombus site (Figure 5A and supplemental Figure 1), but there was no inhibition of thrombus formation. The median platelet thrombus size was comparable after injection of anti-ERp5 antibody or control IgG (data not shown). The same dose of Alexa Fluor 488–labeled control IgG yielded minimal signal at the injury site (Figure 5A). After administration of eptifibatide, ERp5 was not detectable above background (Figure 5B). However, functional studies described in the following section indicate that ERp5 from endothelial cells still contributes to events at the injury site.
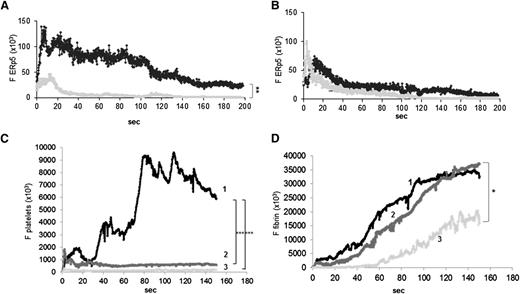
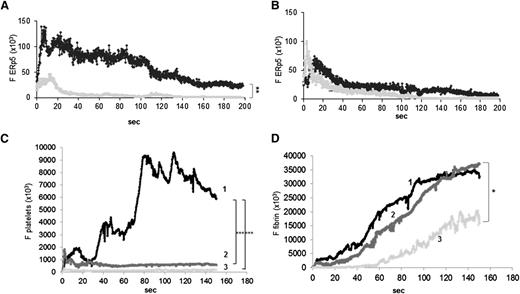
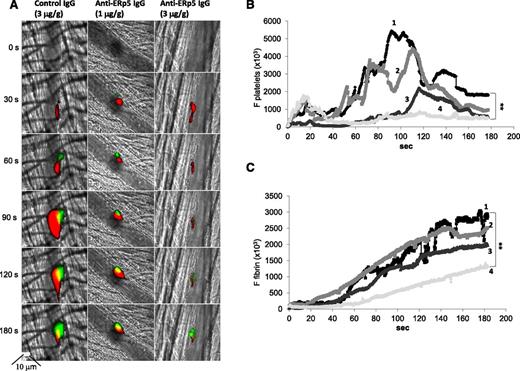
ERp5 is expressed in vivo in the developing thrombus. Anti-ERp5 antibody labeled with Alexa Fluor 488 (0.05 µg/g body weight) or preimmune IgG labeled with Alexa Fluor 488 (0.05 µg/g body weight) and anti CD-42b antibody labeled with DyLight 649 (0.1 µg/g body weight) were infused into a mouse 5 to 10 minutes prior to arteriolar injury. (A) Median total integrated fluorescence for anti-ERp5 (black; 24 thrombi from 3 mice) compared to median of the total integrated fluorescence for control IgG (gray; 27 thrombi from 3 mice); **P < .005. (B) Mice were injected intravenously with eptifibatide (10 µg/g body weight) 10 minutes prior to vessel injury and every 20 minutes after initial injection, and the experiment in panel A was repeated. Median total integrated fluorescence vs time after vessel injury for anti-ERp5 antibody (black) and IgG control (gray) is shown. Platelet accumulation (C) and fibrin generation (D) were measured before (1) and after the injection of eptifibatide alone (2) or eptifibatide followed by anti-ERp5 antibody (3 µg/g body weight) (3). *P < .05;***P < .001. F, median fluorescence intensity.
ERp5 is expressed in vivo in the developing thrombus. Anti-ERp5 antibody labeled with Alexa Fluor 488 (0.05 µg/g body weight) or preimmune IgG labeled with Alexa Fluor 488 (0.05 µg/g body weight) and anti CD-42b antibody labeled with DyLight 649 (0.1 µg/g body weight) were infused into a mouse 5 to 10 minutes prior to arteriolar injury. (A) Median total integrated fluorescence for anti-ERp5 (black; 24 thrombi from 3 mice) compared to median of the total integrated fluorescence for control IgG (gray; 27 thrombi from 3 mice); **P < .005. (B) Mice were injected intravenously with eptifibatide (10 µg/g body weight) 10 minutes prior to vessel injury and every 20 minutes after initial injection, and the experiment in panel A was repeated. Median total integrated fluorescence vs time after vessel injury for anti-ERp5 antibody (black) and IgG control (gray) is shown. Platelet accumulation (C) and fibrin generation (D) were measured before (1) and after the injection of eptifibatide alone (2) or eptifibatide followed by anti-ERp5 antibody (3 µg/g body weight) (3). *P < .05;***P < .001. F, median fluorescence intensity.
Infusion of higher doses of anti-ERp5 antibody inhibits thrombus formation in vivo
Anti-ERp5 antibody (1 and 3 μg/g body weight) or control IgG (3 μg/g body weight) was injected intravenously into C57BL/6 mice, followed by infusion of a monoclonal anti-CD42b antibody labeled with DyLight 649 and a monoclonal anti-fibrin antibody labeled with Alexa Fluor 488. Anti-ERp5 antibody caused a decrease in the deposition of platelets and fibrin in thrombi compared to the infusion of 3 µg/g of preimmune IgG (Figure 6). Platelet thrombus size and fibrin deposition were quantitated as the median area under the curve for their respective fluorescence probes over time after antibody injection (Figure 6B-C). Anti-ERp5 antibody infusion at 3 µg/g body weight caused a 70% decrease in the deposition of platelets and a 62% decrease in fibrin accumulation compared to infusion of 3 µg/g body weight preimmune IgG.
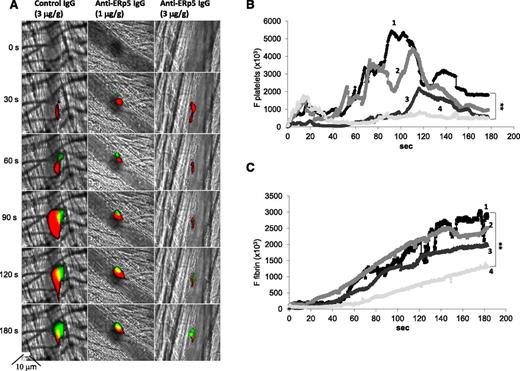
Anti-ERp5 antibody inhibits thrombus formation in vivo. (A) Anti-CD42b antibody labeled with DyLight 649 (0.1 µg/g body weight) and anti-fibrin-specific antibody labeled with Alexa Fluor 488 (0.5 µg/g body weight) were infused into a mouse 5 to 10 minutes prior to arteriolar injury. Preimmune IgG at 3 µg/g body weight (left) or anti-ERp5 antibody at 1 µg/g body weight (middle) and 3 µg/g body weight (right) were infused intravenously 20 minutes prior to injury. Representative binarized images of the fluorescent signal from platelets (red) and fibrin (green) over 180 seconds after laser-induced vessel wall injury show inhibition of thrombus formation with increasing concentrations of anti-ERp5 antibody. Median integrated platelet (B) and fibrin (C) fluorescence over time is shown before (1) vs after infusion of control IgG, 3 µg/g body weight (2); after anti-ERp5 antibody, 1 µg/g body weight (3); and after anti-ERp5 antibody, 3 µg/g body weight (4). **P < .01.
Anti-ERp5 antibody inhibits thrombus formation in vivo. (A) Anti-CD42b antibody labeled with DyLight 649 (0.1 µg/g body weight) and anti-fibrin-specific antibody labeled with Alexa Fluor 488 (0.5 µg/g body weight) were infused into a mouse 5 to 10 minutes prior to arteriolar injury. Preimmune IgG at 3 µg/g body weight (left) or anti-ERp5 antibody at 1 µg/g body weight (middle) and 3 µg/g body weight (right) were infused intravenously 20 minutes prior to injury. Representative binarized images of the fluorescent signal from platelets (red) and fibrin (green) over 180 seconds after laser-induced vessel wall injury show inhibition of thrombus formation with increasing concentrations of anti-ERp5 antibody. Median integrated platelet (B) and fibrin (C) fluorescence over time is shown before (1) vs after infusion of control IgG, 3 µg/g body weight (2); after anti-ERp5 antibody, 1 µg/g body weight (3); and after anti-ERp5 antibody, 3 µg/g body weight (4). **P < .01.
Infusion of eptifibatide diminished platelet thrombus formation, as expected, but fibrin deposition was similar in the absence and presence of eptifibatide (Figure 5C-D). Infusion of both eptifibatide and anti-ERp5 antibody resulted in a 68% reduction of fibrin deposition compared to infusion of eptifibatide alone (Figure 5D). These results indicate that endothelial-derived ERp5 contributes to fibrin thrombus formation in vivo.
ERp5 binds directly to αIIbβ3
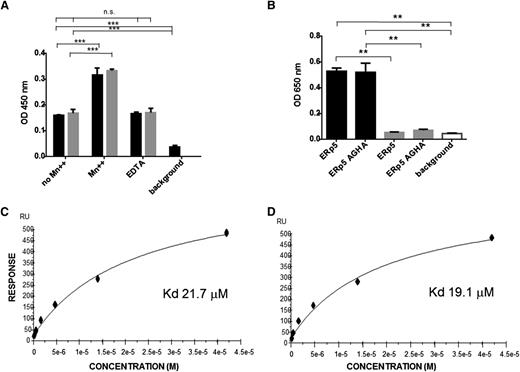
To remain at the site of thrombus formation in the environment of flowing blood, ERp5 must bind to a surface at the injury site. Because it has been previously reported that ERp5 coimmunoprecipitates with αIIbβ3 from thrombin-activated platelets, we examined the binding of ERp5 to αIIbβ3.25 In the presence of EDTA or in the absence of manganese ion (Mn++), we observed equivalent binding between ERp5 and αIIbβ3 (Figure 7A). This binding increased in the presence of Mn++, a feature observed for this integrin’s primary ligand, fibrinogen (Figure 7A).33 Similarly, the inactive form of ERp5 (ERp5-AGHA, which contains the mutated active sites in the a and a′ domains) bound equivalently to αIIbβ3 in the presence of EDTA or in the absence of Mn++. These results indicate that the active-site motif CXXC is not required for binding to αIIbβ3.
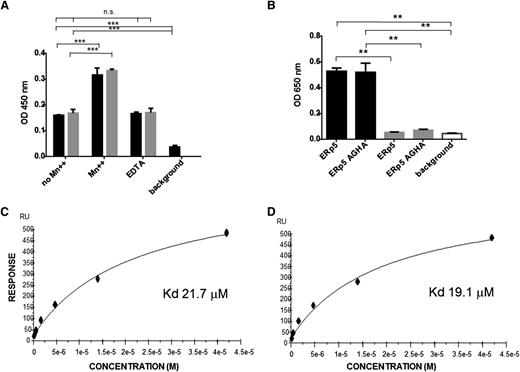
Binding of ERp5 and ERp5-AGHA to αIIbβ3 and directly to the β3 subunit. (A) Binding of αIIbβ3 to immobilized ERp5 or ERp5-AGHA was measured in the presence or absence of MnCl2 (Mn++; 2 mM), in the presence of EDTA (5 mM), or in the absence of added αIIbβ3 (background), as indicated. Bound αIIbβ3 was detected with an anti-CD41 conformation-independent antibody. ERp5 (black) and ERp5-AGHA (gray) binding to αIIbβ3 were compared by nonparametric Student t tests: no Mn++ vs Mn++ and no Mn++ vs background,***P < .0001. There is no significant difference between no Mn++ vs EDTA. Results are the average of 3 experiments. (B) Binding of ERp5 (100 nM) or ERp5-AGHA (100 nM) to αIIbβ3 (black) or glycoprotein (GP)Ibα (gray), both coated at 20 nM on 96-well plates in the absence of Mn++ or in the absence of bound ERp5 or ERp5-AGHA (background; white). Bound ERp5 or ERp5-AGHA was detected with anti-ERp5 antibody. ERp5 binding to αIIbβ3 vs GPIba, ERp5-AGHA binding to αIIbβ3 vs GPIbα, ERp5 binding to αIIbβ3 vs background, and ERp5-AGHA binding to αIIbβ3 vs background, **P < .005. Binding of ERp5 or ERp5-AGHA to GPIbα vs background was not statistically significant. Results are the average of 3 experiments. Surface plasmon resonance was used to determine the binding constant for ERp5 with recombinant β3 integrin tagged with calmodulin in the presence (C) or absence of Mn++ (D). ERp5 was coated on the Biacore chip. Lines represent the fitted curves of ERp5 at concentrations of 0, 0.33, 0.66, 1.31, 2.63, 5.25, 10.25, 21, and 42 µM. The KD values for ERp5 interaction with the β3 subunit in the presence and absence of Mn++ were 21.7 μM and 19.1 μM, respectively. ERp5 did not bind to calmodulin alone. n.s., not significant; RU, relative units.
Binding of ERp5 and ERp5-AGHA to αIIbβ3 and directly to the β3 subunit. (A) Binding of αIIbβ3 to immobilized ERp5 or ERp5-AGHA was measured in the presence or absence of MnCl2 (Mn++; 2 mM), in the presence of EDTA (5 mM), or in the absence of added αIIbβ3 (background), as indicated. Bound αIIbβ3 was detected with an anti-CD41 conformation-independent antibody. ERp5 (black) and ERp5-AGHA (gray) binding to αIIbβ3 were compared by nonparametric Student t tests: no Mn++ vs Mn++ and no Mn++ vs background,***P < .0001. There is no significant difference between no Mn++ vs EDTA. Results are the average of 3 experiments. (B) Binding of ERp5 (100 nM) or ERp5-AGHA (100 nM) to αIIbβ3 (black) or glycoprotein (GP)Ibα (gray), both coated at 20 nM on 96-well plates in the absence of Mn++ or in the absence of bound ERp5 or ERp5-AGHA (background; white). Bound ERp5 or ERp5-AGHA was detected with anti-ERp5 antibody. ERp5 binding to αIIbβ3 vs GPIba, ERp5-AGHA binding to αIIbβ3 vs GPIbα, ERp5 binding to αIIbβ3 vs background, and ERp5-AGHA binding to αIIbβ3 vs background, **P < .005. Binding of ERp5 or ERp5-AGHA to GPIbα vs background was not statistically significant. Results are the average of 3 experiments. Surface plasmon resonance was used to determine the binding constant for ERp5 with recombinant β3 integrin tagged with calmodulin in the presence (C) or absence of Mn++ (D). ERp5 was coated on the Biacore chip. Lines represent the fitted curves of ERp5 at concentrations of 0, 0.33, 0.66, 1.31, 2.63, 5.25, 10.25, 21, and 42 µM. The KD values for ERp5 interaction with the β3 subunit in the presence and absence of Mn++ were 21.7 μM and 19.1 μM, respectively. ERp5 did not bind to calmodulin alone. n.s., not significant; RU, relative units.
ERp5 binds directly to β3 integrin
The KD for ERp5 binding to immobilized β3 integrin was determined by surface plasmon resonance. ERp5 in solution binds β3 integrin in the presence (Figure 7C) or absence of Mn++ (Figure 7D), with a KD value of 21.7 µM or 19.1 µM, respectively. Because the β3 subunit was expressed linked to calmodulin, we confirmed that ERp5 does not bind to calmodulin alone using surface plasmon resonance (data not shown).
Discussion
Anti-ERp5 antibodies have been previously shown to inhibit platelet aggregation, fibrinogen binding, and P-selectin expression on platelet activation in vitro.25 In the current study, we report that ERp5 is released in vitro from platelets and endothelial cells and accumulates in vivo at sites of laser injury in a mouse model of thrombosis. The enzyme is released and retained at sites of vascular injury in vivo, promoting thrombus development. When platelet thrombus formation is inhibited with eptifibatide, ERp5 released from endothelial cells supports fibrin generation in response to a laser-induced injury. ERp5 binds directly to the calcium ion–stabilized β subunit of the fibrinogen receptor αIIbβ3, a likely mechanism by which ERp5 remains associated with the platelet thrombus.
Two other thiol isomerases are known to play a role in thrombus formation. PDI is essential for platelet thrombus formation and fibrin generation in vivo.16-18,34 ERp57 also mediates platelet thrombus formation in mouse thrombosis models.21,22 A growing body of data indicates that ERp5, PDI, and ERp57 have independent roles in promoting thrombus formation. Platelets from mice that have a platelet-specific deficiency in PDI exhibit reduced response to low to intermediate concentrations of thrombin.34 Treatment of platelets from these animals with inhibitory anti-ERp57 antibodies led to further decreases in this response. Similarly, in vitro studies treating platelets with collagen in the presence of a combination of inhibitory antibodies to both ERp5 and PDI showed an additive, but not a synergistic, effect in the inhibition of platelet aggregation, activation, and fibrinogen binding to platelets compared to collagen-stimulated platelets in the presence of anti-PDI alone.25 These data suggest that ERp5, ERp57, and PDI play distinct roles in platelet aggregation, adenosine triphosphate secretion, and αIIbβ3 activation. Indeed, there is a striking difference between mice with PDI-null platelets and those with ERp57-null platelets. Platelet deficiency of either thiol isomerase leads to decreased thrombus formation; however, mice with PDI-null platelets exhibit normal tail bleeding times, whereas mice with ERp57-null platelets exhibit tail bleeding times prolonged twofold over wild-type mice.22,34 These results further suggest that individual thiol isomerases may play distinct roles in hemostasis and thrombosis.
In the presence of eptifibatide to prevent platelet thrombus formation, the level of ERp5 antigen observed at sites of laser-induced injury is below the level of detection using our intravital microscopy system. However, functional studies indicate that ERp5 enzymatic activity is present at the injury site under these conditions because fibrin generation is equivalent in the absence or presence of the eptifibatide alone but inhibited in the presence of both eptifibatide and anti-ERp5 antibody. This result is similar to that observed when the presence of PDI was examined at sites of laser-induced thrombus formation in eptifibatide-treated mice: the level of PDI was reduced at the thrombus site, but fibrin generation was normal.17 Although initial platelet deposition on the injured vessel wall could be a source of ERp5 or PDI in eptifibatide-treated animals, endothelial cells are the more likely source under these experimental conditions. This conclusion is supported by results observed in platelet-specific PDI conditional knockout mice.34 In these mice, both initial platelet deposition on the vessel wall and fibrin generation are equivalent to wild-type mice, whereas a platelet thrombus never develops in response to laser-induced injury. However, in conditional knockout mice, the platelets being deposited at the injured vessel wall lack PDI and cannot be the source of this enzyme observed at the injury site.
Binding of ERp5 to soluble αIIbβ3 is increased in the presence of Mn++, a potent allosteric integrin activator, as has been described for ERp57 and PDI.22,35 Mn++ enhances binding of αIIbβ3 to fibrinogen by increasing the binding energy and by decreasing the off-rate for this interaction.36 It is not known how Mn++ effects the binding of αIIbβ3 to the thiol isomerases, but a similar mechanism may apply. The association of ERp5 to αIIbβ3 is not mediated through the active-site cysteines because the active-site variant ERp5-AGHA, lacking the CGHC motif, exhibits binding properties similar to those of the wild-type enzyme. In a parallel manner, the binding of PDI to β2 integrin of neutrophils was attributed to electrostatic interaction but not to disulfide bonding.12
PDI, ERp57, and ERp5 have all been implicated in regulating the activation state of αIIbβ3 in vitro.9,10,25,34,37,38 The question arises whether the roles of these 3 enzymes in β3 integrin activation are redundant or whether they have unique functions in this process. A mechanism for the participation of all of these thiol isomerases in substrate activation, including αIIbβ3, is suggested by an elegant study of polyomavirus infection. This study demonstrated that 3 thiol isomerases coordinately catalyze the unfolding of C-terminal arm of VP1, the major coat protein of the virus, to facilitate infection.39 Alternatively or additionally, each of these thiol isomerases may interact with a unique set of substrates. The β3 integrins may facilitate the roles of ERp5, ERp57, and PDI in thrombus formation by providing a platform for formation of complexes with these enzymes and their thrombosis-relevant substrates on the surface of activated platelets or endothelial cells at sites of vascular injury.
The participation of the thiol isomerases ERp5, ERp57, and PDI in thrombosis may make these enzymes attractive drug targets for novel antithrombotics. Indeed, we have recently shown that low molecular weight inhibitors of PDI represent a potential new class of antithrombotic agents.40,41 Similar agents against ERp5 may also prove efficacious.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jonathan Gibbins (University of Reading) for the GST-ERp5 expression plasmid and Dr Willem Ouwehand (University of Cambridge) for calmodulin-tagged β3-expressing cells.
This study was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (PO1 HL087203, U54 HL112302, R01 HL092125, and T32 HL007917) and a Research Award (F.H.P.) from the St George Medical Research Foundation, Australia.
Authorship
Contribution: F.H.P. designed and performed the experiments, analyzed the data, and wrote the manuscript; L.L., S.G., L.B.-M., and J.D.S. designed and performed the experiments and generated the reagents; M.H. contributed to the study design; and B.F. and B.C.F. initiated the studies, designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce Furie and Barbara Furie, Beth Israel Deaconess Medical Center, 330 Brookline Ave, E/CLS 903, Boston, MA 02215; e-mail: bfurie@bidmc.harvard.edu.